Page 563 - Corrosion Engineering Principles and Practice
P. 563
526 C h a p t e r 1 3 C a t h o d i c P r o t e c t i o n 527
60
50
Cumulative corrosion leaks 30 CP restored
40
CP restored
CP interrupted
20
10 CP interrupted
CP first installed-1934
0
1930 1940 1950 1960 1970 1980 1990 2000
Year
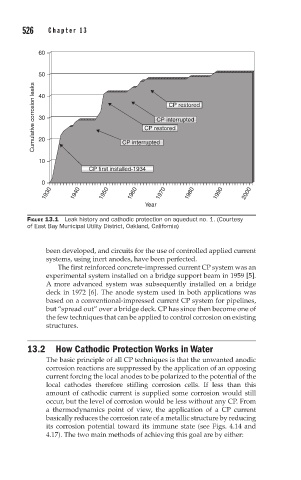
FIGURE 13.1 Leak history and cathodic protection on aqueduct no. 1. (Courtesy
of East Bay Municipal Utility District, Oakland, California)
been developed, and circuits for the use of controlled applied current
systems, using inert anodes, have been perfected.
The first reinforced concrete-impressed current CP system was an
experimental system installed on a bridge support beam in 1959 [5].
A more advanced system was subsequently installed on a bridge
deck in 1972 [6]. The anode system used in both applications was
based on a conventional-impressed current CP system for pipelines,
but “spread out” over a bridge deck. CP has since then become one of
the few techniques that can be applied to control corrosion on existing
structures.
13.2 How Cathodic Protection Works in Water
The basic principle of all CP techniques is that the unwanted anodic
corrosion reactions are suppressed by the application of an opposing
current forcing the local anodes to be polarized to the potential of the
local cathodes therefore stifling corrosion cells. If less than this
amount of cathodic current is supplied some corrosion would still
occur, but the level of corrosion would be less without any CP. From
a thermodynamics point of view, the application of a CP current
basically reduces the corrosion rate of a metallic structure by reducing
its corrosion potential toward its immune state (see Figs. 4.14 and
4.17). The two main methods of achieving this goal are by either: