Page 570 - Corrosion Engineering Principles and Practice
P. 570
532 C h a p t e r 1 3 C a t h o d i c P r o t e c t i o n 533
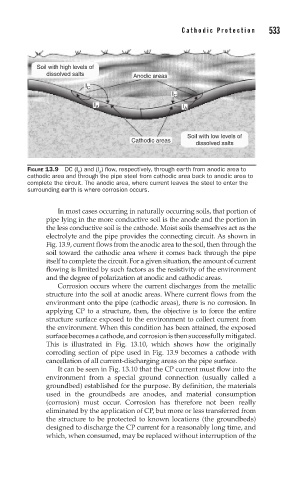
Soil with high levels of
dissolved salts Anodic areas
I C
I C
I A I A
Soil with low levels of
Cathodic areas dissolved salts
FIGURE 13.9 DC (I ) and (I ) flow, respectively, through earth from anodic area to
A
C
cathodic area and through the pipe steel from cathodic area back to anodic area to
complete the circuit. The anodic area, where current leaves the steel to enter the
surrounding earth is where corrosion occurs.
In most cases occurring in naturally occurring soils, that portion of
pipe lying in the more conductive soil is the anode and the portion in
the less conductive soil is the cathode. Moist soils themselves act as the
electrolyte and the pipe provides the connecting circuit. As shown in
Fig. 13.9, current flows from the anodic area to the soil, then through the
soil toward the cathodic area where it comes back through the pipe
itself to complete the circuit. For a given situation, the amount of current
flowing is limited by such factors as the resistivity of the environment
and the degree of polarization at anodic and cathodic areas.
Corrosion occurs where the current discharges from the metallic
structure into the soil at anodic areas. Where current flows from the
environment onto the pipe (cathodic areas), there is no corrosion. In
applying CP to a structure, then, the objective is to force the entire
structure surface exposed to the environment to collect current from
the environment. When this condition has been attained, the exposed
surface becomes a cathode, and corrosion is then successfully mitigated.
This is illustrated in Fig. 13.10, which shows how the originally
corroding section of pipe used in Fig. 13.9 becomes a cathode with
cancellation of all current-discharging areas on the pipe surface.
It can be seen in Fig. 13.10 that the CP current must flow into the
environment from a special ground connection (usually called a
groundbed) established for the purpose. By definition, the materials
used in the groundbeds are anodes, and material consumption
(corrosion) must occur. Corrosion has therefore not been really
eliminated by the application of CP, but more or less transferred from
the structure to be protected to known locations (the groundbeds)
designed to discharge the CP current for a reasonably long time, and
which, when consumed, may be replaced without interruption of the