Page 86 - Corrosion Engineering Principles and Practice
P. 86
62 C h a p t e r 4 C o r r o s i o n T h e r m o d y n a m i c s 63
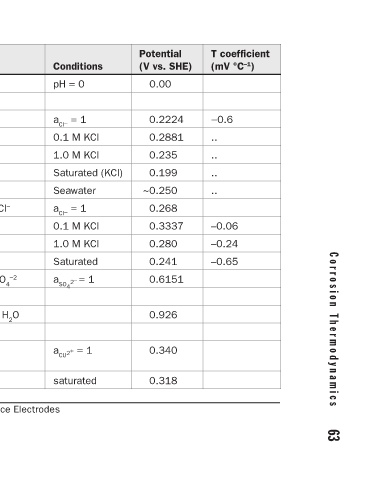
T coefficient (mV °C –1 ) −0.6 .. .. .. .. –0.06 –0.24 –0.65
Potential (V vs. SHE) 0.00 0.2224 0.2881 0.235 0.199 ~0.250 0.268 0.3337 0.280 0.241 0.6151 0.926 0.340 0.318
Conditions pH = 0 a Cl − = 1 0.1 M KCl 1.0 M KCl Saturated (KCl) Seawater a Cl − = 1 0.1 M KCl 1.0 M KCl Saturated a SO 4 2 – = 1 a CU2 + = 1 saturated
−2
Equilibrium reaction and Nernst equation 2 H + + 2 e − = H 2 E o − 0.059 pH AgCl + e − = Ag + Cl − E o − 0.059 log 10 a Cl − Hg 2 Cl 2 + 2 e − = 2 Hg + 2 Cl − E o − 0.059 log 10 a Cl − Hg 2 SO 4 + 2 e − = 2 Hg + SO 4 E o − 0.0295 log 10 a SO 4 2 – HgO + 2 e − + 2 H + = Hg + H 2 O E o − 0.
Standard hydrogen electrode (SHE)
Name Silver chloride Calomel Mercurous sulfate Mercuric oxide Copper sulfate TABLE 4.7