Page 91 - Corrosion Engineering Principles and Practice
P. 91
66 C h a p t e r 4 C o r r o s i o n T h e r m o d y n a m i c s 67
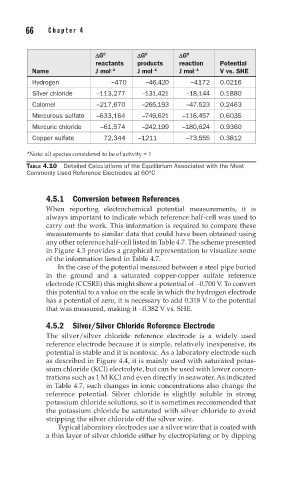
DG DG DG
0
0
0
reactants products reaction Potential
Name J mol –1 J mol –1 J mol –1 V vs. SHE
Hydrogen –470 –46,420 –4172 0.0216
Silver chloride –113,277 –131,421 –18,144 0.1880
Calomel –217,670 –265,193 –47,523 0.2463
Mercurous sulfate –633,164 –749,621 –116,457 0.6035
Mercuric chloride –61,574 –242,199 –180,624 0.9360
Copper sulfate 72,344 –1211 –73,555 0.3812
*Note: all species considered to be of activity = 1
TABLE 4.10 Detailed Calculations of the Equilibrium Associated with the Most
Commonly Used Reference Electrodes at 60°C
4.5.1 Conversion between References
When reporting electrochemical potential measurements, it is
always important to indicate which reference half-cell was used to
carry out the work. This information is required to compare these
measurements to similar data that could have been obtained using
any other reference half-cell listed in Table 4.7. The scheme presented
in Figure 4.3 provides a graphical representation to visualize some
of the information listed in Table 4.7.
In the case of the potential measured between a steel pipe buried
in the ground and a saturated copper-copper sulfate reference
electrode (CCSRE) this might show a potential of −0.700 V. To convert
this potential to a value on the scale in which the hydrogen electrode
has a potential of zero, it is necessary to add 0.318 V to the potential
that was measured, making it −0.382 V vs. SHE.
4.5.2 Silver/Silver Chloride Reference Electrode
The silver/silver chloride reference electrode is a widely used
reference electrode because it is simple, relatively inexpensive, its
potential is stable and it is nontoxic. As a laboratory electrode such
as described in Figure 4.4, it is mainly used with saturated potas-
sium chloride (KCl) electrolyte, but can be used with lower concen-
trations such as 1 M KCl and even directly in seawater. As indicated
in Table 4.7, such changes in ionic concentrations also change the
reference potential. Silver chloride is slightly soluble in strong
potassium chloride solutions, so it is sometimes recommended that
the potassium chloride be saturated with silver chloride to avoid
stripping the silver chloride off the silver wire.
Typical laboratory electrodes use a silver wire that is coated with
a thin layer of silver chloride either by electroplating or by dipping