Page 90 - Corrosion Engineering Principles and Practice
P. 90
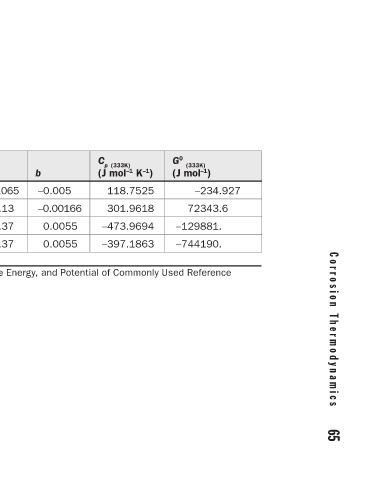
64 C h a p t e r 4 C o r r o s i o n T h e r m o d y n a m i c s 65
–234.927 72343.6
G 0 (333K) ) (J mol –1 –129881. –744190.
C p (333K) K –1 ) (J mol –1 118.7525 301.9618 –473.9694 –397.1863
–0.005 –0.00166 0.0055 0.0055
b
0.065 0.13 –0.37 –0.37 Thermodynamic Data for Soluble Species, the Free Energy, and Potential of Commonly Used Reference
a
Š (298 K) (J mol –1 ) –20.9 –249.04 8.32 52.592
S 0 (298 K) ) (J mol –1 0 –207.2 –12.6 10.752
G 0 (298 K) ) (J mol –1 0 65689 –131260 –744600
Species H + Cu 2+ Cl − 2− SO 4 TABLE 4.9 Electrodes at 60°C