Page 93 - Corrosion Engineering Principles and Practice
P. 93
68 C h a p t e r 4 C o r r o s i o n T h e r m o d y n a m i c s 69
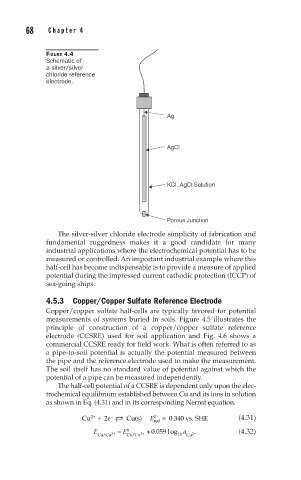
FIGURE 4.4
Schematic of
a silver/silver
chloride reference
electrode.
Ag
AgCl
KCl, AgCl Solution
Porous Junction
The silver-silver chloride electrode simplicity of fabrication and
fundamental ruggedness makes it a good candidate for many
industrial applications where the electrochemical potential has to be
measured or controlled. An important industrial example where this
half-cell has become indispensable is to provide a measure of applied
potential during the impressed current cathodic protection (ICCP) of
sea-going ships.
4.5.3 Copper/Copper Sulfate Reference Electrode
Copper/copper sulfate half-cells are typically favored for potential
measurements of systems buried in soils. Figure 4.5 illustrates the
principle of construction of a copper/copper sulfate reference
electrode (CCSRE) used for soil application and Fig. 4.6 shows a
commercial CCSRE ready for field work. What is often referred to as
a pipe-to-soil potential is actually the potential measured between
the pipe and the reference electrode used to make the measurement.
The soil itself has no standard value of potential against which the
potential of a pipe can be measured independently.
The half-cell potential of a CCSRE is dependent only upon the elec-
trochemical equilibrium established between Cu and its ions in solution
as shown in Eq. (4.31) and in its corresponding Nernst equation.
Cu 2+ + 2e Cu(s) E 0 red = 0.340 vs. SHE (4.31)
−
E 2+ = E 0 2+ + 0 05. 9 log a 2+ (4.32)
Cu/Cu Cu/Cu 10 Cu