Page 95 - Corrosion Engineering Principles and Practice
P. 95
70 C h a p t e r 4 C o r r o s i o n T h e r m o d y n a m i c s 71
Calculation Example
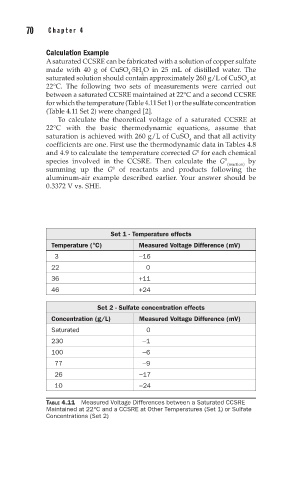
A saturated CCSRE can be fabricated with a solution of copper sulfate
made with 40 g of CuSO ·5H O in 25 mL of distilled water. The
2
4
saturated solution should contain approximately 260 g/L of CuSO at
4
22°C. The following two sets of measurements were carried out
between a saturated CCSRE maintained at 22°C and a second CCSRE
for which the temperature (Table 4.11 Set 1) or the sulfate concentration
(Table 4.11 Set 2) were changed [2].
To calculate the theoretical voltage of a saturated CCSRE at
22°C with the basic thermodynamic equations, assume that
saturation is achieved with 260 g/L of CuSO and that all activity
4
coefficients are one. First use the thermodynamic data in Tables 4.8
and 4.9 to calculate the temperature corrected G for each chemical
0
species involved in the CCSRE. Then calculate the G 0 (reaction) by
summing up the G of reactants and products following the
0
aluminum-air example described earlier. Your answer should be
0.3372 V vs. SHE.
Set 1 - Temperature effects
Temperature (°C) Measured Voltage Difference (mV)
3 −16
22 0
36 +11
46 +24
Set 2 - Sulfate concentration effects
Concentration (g/L) Measured Voltage Difference (mV)
Saturated 0
230 −1
100 −6
77 −9
26 −17
10 −24
TABLE 4.11 Measured Voltage Differences between a Saturated CCSRE
Maintained at 22°C and a CCSRE at Other Temperatures (Set 1) or Sulfate
Concentrations (Set 2)