Page 202 - Earth's Climate Past and Future
P. 202
178 PART III • Orbital-Scale Climate Change
380 in Earth’s natural reservoirs and the transfers among
them (Figure 10–5). In Part II, we focused on the very
slow exchanges between the carbon buried in Earth’s sed-
360 iments and rocks and the carbon stored in Earth’s surface
reservoirs (the atmosphere, vegetation and ocean). Over
Instrumental many millions of years, the cumulative effects of these
340 measurements
slow exchanges caused large changes in CO .
2
CO 2 (ppm) 320 changes in CO and CH that occur over thousands to
In this chapter, we are interested in orbital-scale
4
2
tens of thousands of years. These faster changes can be
Ice core explained only by rapid exchanges of carbon among the
300 surface reservoirs (see Figure 3–3). Large amounts of
measurements
carbon must have moved among these reservoirs during
the length of an orbital cycle.
280
To explore how carbon has moved among these
1225–1560 1700 1800 1900 2000 reservoirs, we need a quantitative way to track its move-
Year ment. Fortunately, two carbon isotopes exist in nature,
A CO
2 and different types of carbon in the climate system have
13
distinctive carbon isotope (δ C) ratios that give scien-
1800 tists a way of tracking how carbon has moved among
these reservoirs (Appendix 2).
1600 Instrumental Most carbon occurs in oxygen-rich environments in
measurements the atmosphere, oceans, and vegetation. Carbon moves
among these reservoirs in one of two forms: organic
CH 4 (ppb) 1400 carbon, which includes both living and dead organic
matter, and inorganic carbon, which consists mainly of
1200
–1
–2
3
includes CO in the atmosphere (companion Web site,
1000 Ice core ions dissolved in water (HCO 3 and CO ) but also
2
measurements 13
pp. 30–33). Abundances and typical δ C values of
800 organic and inorganic carbon in the major reservoirs
are shown in Figure 10–5.
13
1700 1800 1900 2000 The δ C values of inorganic and organic carbon
Year differ mainly because of changes that occur during pho-
B CH 4 tosynthesis, a process by which plants create organic
carbon from inorganic sources (companion Web site,
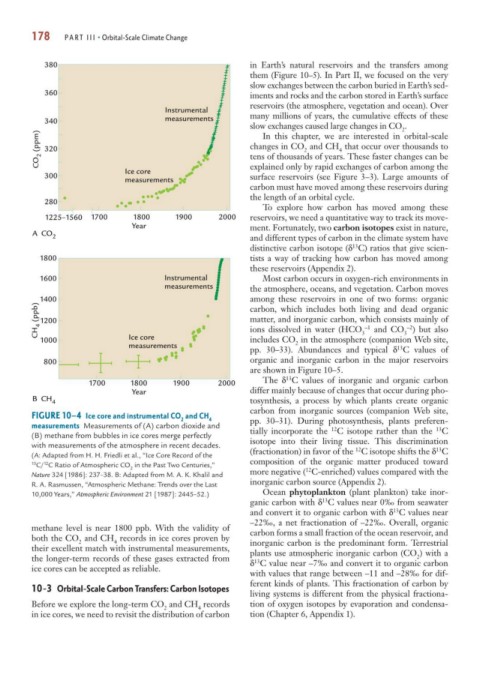
FIGURE 10–4 Ice core and instrumental CO and CH
2 4 pp. 30–31). During photosynthesis, plants preferen-
measurements Measurements of (A) carbon dioxide and tially incorporate the C isotope rather than the C
13
12
(B) methane from bubbles in ice cores merge perfectly
with measurements of the atmosphere in recent decades. isotope into their living tissue. This discrimination
(fractionation) in favor of the C isotope shifts the δ C
13
12
(A: Adapted from H. H. Friedli et al., “Ice Core Record of the
12
13 C/ C Ratio of Atmospheric CO in the Past Two Centuries,” composition of the organic matter produced toward
2 12
Nature 324 [1986]: 237–38. B: Adapted from M. A. K. Khalil and more negative ( C-enriched) values compared with the
R. A. Rasmussen, “Atmospheric Methane: Trends over the Last inorganic carbon source (Appendix 2).
10,000 Years,” Atmospheric Environment 21 [1987]: 2445–52.) Ocean phytoplankton (plant plankton) take inor-
13
ganic carbon with δ C values near 0‰ from seawater
13
and convert it to organic carbon with δ C values near
–22‰, a net fractionation of –22‰. Overall, organic
methane level is near 1800 ppb. With the validity of carbon forms a small fraction of the ocean reservoir, and
both the CO and CH records in ice cores proven by
2 4 inorganic carbon is the predominant form. Terrestrial
their excellent match with instrumental measurements, plants use atmospheric inorganic carbon (CO ) with a
2
the longer-term records of these gases extracted from δ C value near –7‰ and convert it to organic carbon
13
ice cores can be accepted as reliable.
with values that range between –11 and –28‰ for dif-
ferent kinds of plants. This fractionation of carbon by
10-3 Orbital-Scale Carbon Transfers: Carbon Isotopes
living systems is different from the physical fractiona-
Before we explore the long-term CO and CH records tion of oxygen isotopes by evaporation and condensa-
2 4
in ice cores, we need to revisit the distribution of carbon tion (Chapter 6, Appendix 1).