Page 138 - Electrical Properties of Materials
P. 138
120 The band theory of solids
(a) The detailed continuation of the story depends on the variation of energy
π/a
with k in the higher band, but one thing is certain: the higher band will not be
empty.
k For an atom with two electrons, the number of available states is equal to
y
the number of electrons. If the energy gap is large (say, two units instead of
0 half a unit), then all the states in the rectangle would be filled, and the material
would be an insulator. If the energy gap is small (half a unit in our example),
then some states will remain unfilled in the rectangle, and some states will be
filled in the higher band. This means that both bands will contribute to elec-
trical conduction. There will be holes coming from the rectangle and electrons
–π/a
– π 0 k x π from the higher band. This is how it happens that in some metals holes are the
a a
dominant charge-carriers.
(b) 7.11 Finite temperatures
All we said so far applies to zero temperature. What happens at finite
temperatures? And what is particularly important, what happens at about room
k
y temperature, at which most electronic devices are supposed to work?
For finite temperatures it is no longer valid to assume that all states up to
the Fermi energy are filled and all states above that are empty. The demarcation
line between filled and unfilled states will become less sharp.
Let us see first what happens to a metal. Its highest energy band is about
half-filled at absolute zero; at higher temperatures some of the electrons will
acquire somewhat higher energies in the band, but that is all. There will be very
π 0 k π
– x little change in the effective number of electrons. A metal will stay a metal at
a a
higher temperatures.
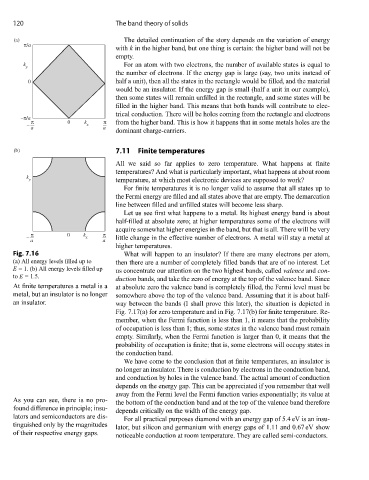
Fig. 7.16 What will happen to an insulator? If there are many electrons per atom,
(a) All energy levels filled up to then there are a number of completely filled bands that are of no interest. Let
E = 1. (b) All energy levels filled up us concentrate our attention on the two highest bands, called valence and con-
to E = 1.5.
duction bands, and take the zero of energy at the top of the valence band. Since
At finite temperatures a metal is a at absolute zero the valence band is completely filled, the Fermi level must be
metal, but an insulator is no longer somewhere above the top of the valence band. Assuming that it is about half-
an insulator. way between the bands (I shall prove this later), the situation is depicted in
Fig. 7.17(a) for zero temperature and in Fig. 7.17(b) for finite temperature. Re-
member, when the Fermi function is less than 1, it means that the probability
of occupation is less than 1; thus, some states in the valence band must remain
empty. Similarly, when the Fermi function is larger than 0, it means that the
probability of occupation is finite; that is, some electrons will occupy states in
the conduction band.
We have come to the conclusion that at finite temperatures, an insulator is
no longer an insulator. There is conduction by electrons in the conduction band,
and conduction by holes in the valence band. The actual amount of conduction
depends on the energy gap. This can be appreciated if you remember that well
away from the Fermi level the Fermi function varies exponentially; its value at
As you can see, there is no pro-
the bottom of the conduction band and at the top of the valence band therefore
found difference in principle; insu-
depends critically on the width of the energy gap.
lators and semiconductors are dis-
For all practical purposes diamond with an energy gap of 5.4 eV is an insu-
tinguished only by the magnitudes
lator, but silicon and germanium with energy gaps of 1.11 and 0.67 eV show
of their respective energy gaps.
noticeable conduction at room temperature. They are called semi-conductors.