Page 245 - Electrical Properties of Materials
P. 245
Types of polarization 227
For low electric fields, we may assume that the dipole moment is propor-
tional to the local electric field, E : α is a constant called the polariz-
ability.
μ = αE . (10.8)
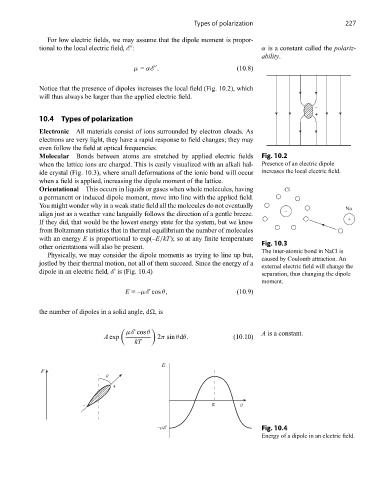
Notice that the presence of dipoles increases the local field (Fig. 10.2), which
will thus always be larger than the applied electric field.
_
+
10.4 Types of polarization
Electronic All materials consist of ions surrounded by electron clouds. As
electrons are very light, they have a rapid response to field changes; they may
even follow the field at optical frequencies.
Molecular Bonds between atoms are stretched by applied electric fields Fig. 10.2
when the lattice ions are charged. This is easily visualized with an alkali hal- Presence of an electric dipole
ide crystal (Fig. 10.3), where small deformations of the ionic bond will occur increases the local electric field.
when a field is applied, increasing the dipole moment of the lattice.
Orientational This occurs in liquids or gases when whole molecules, having Cl
a permanent or induced dipole moment, move into line with the applied field.
You might wonder why in a weak static field all the molecules do not eventually
– Na
align just as a weather vane languidly follows the direction of a gentle breeze.
+
If they did, that would be the lowest energy state for the system, but we know
from Boltzmann statistics that in thermal equilibrium the number of molecules
with an energy E is proportional to exp(–E/kT); so at any finite temperature
Fig. 10.3
other orientations will also be present.
The inter-atomic bond in NaCl is
Physically, we may consider the dipole moments as trying to line up but,
caused by Coulomb attraction. An
jostled by their thermal motion, not all of them succeed. Since the energy of a
external electric field will change the
dipole in an electric field, E is (Fig. 10.4) separation, thus changing the dipole
moment.
E =–μE cos θ, (10.9)
the number of dipoles in a solid angle, d ,is
μE cos θ A is a constant.
A exp 2π sin θdθ. (10.10)
kT
E
θ
+
– π θ
–μ Fig. 10.4
Energy of a dipole in an electric field.