Page 394 - Electrical Properties of Materials
P. 394
376 Superconductivity
a second electron of opposite spin. It has been suggested recently that induced
magnetic fluctuations may also be responsible for the pairing mechanism.
14.4 Thermodynamical treatment
Let us look again at Fig. 14.4. Above the curve our substance behaves in the
normally accepted way. It has the same sort of properties it had at room tem-
perature. Its magnetic properties are the same, and its electric properties are
the same; true, the electrical resistivity is smaller than at room temperature,
but there is nothing unexpected in that. However, as soon as we cross the
curve, the properties of the substance become qualitatively different. Above
the curve the substance is non-magnetic, below the curve it becomes diamag-
netic; above the curve it has a finite electrical resistance, below the curve the
electric resistance is zero.
If you think about it a little you will see that the situation is very similar to
what you have studied under the name of ‘phase change’ or ‘phase transition’
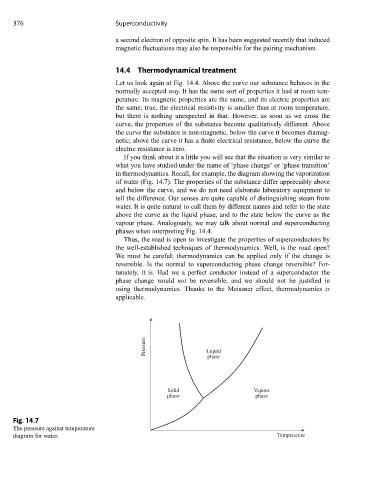
in thermodynamics. Recall, for example, the diagram showing the vaporization
of water (Fig. 14.7). The properties of the substance differ appreciably above
and below the curve, and we do not need elaborate laboratory equipment to
tell the difference. Our senses are quite capable of distinguishing steam from
water. It is quite natural to call them by different names and refer to the state
above the curve as the liquid phase, and to the state below the curve as the
vapour phase. Analogously, we may talk about normal and superconducting
phases when interpreting Fig. 14.4.
Thus, the road is open to investigate the properties of superconductors by
the well-established techniques of thermodynamics. Well, is the road open?
We must be careful; thermodynamics can be applied only if the change is
reversible. Is the normal to superconducting phase change reversible? For-
tunately, it is. Had we a perfect conductor instead of a superconductor the
phase change would not be reversible, and we should not be justified in
using thermodynamics. Thanks to the Meissner effect, thermodynamics is
applicable.
Pressure Liquid
phase
Solid Vapour
phase phase
Fig. 14.7
The pressure against temperature
diagram for water. Temperature