Page 299 - Elements of Chemical Reaction Engineering 3rd Edition
P. 299
Chap. 5 Questions and Problems
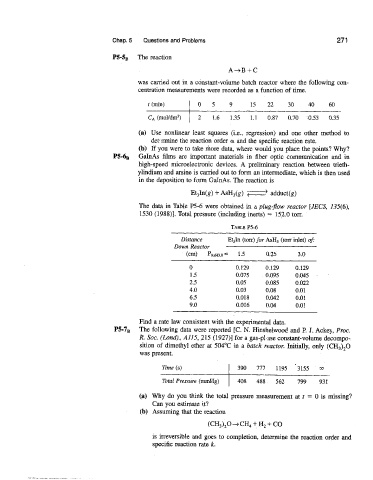
P5-S8, The reaction
was carried out in a constant-volume batch reactor where the followin,g con-
centration measurements were recorded as a function of time.
t (min) 0 5 9 15 22 30 40 610
- -
C,4(mol/dm3) 2 1.6 1.35 1.1 0.87 0.70 -0.53 0.35
(a) Use nonlinear least squares (i.e., regression) and one other method to
de1t:nnine the reaction order a and the specific reaction rate.
(b) If :you were to take more data, where would you place the points? Why?
P5-6, GaInAs films are iniportant materials in fiber optic communication and in
high-speed microelectronic devices. A prelirriinary reaction between trieth-
ylindiurn and arsine is carried out to form an intermediate, which is then used
in the deposition to form GaInAs. The reaction is
The data in Table P5-6 were obtained in a plug-flow reactor [JECS, 1.35(6),
1530 (1988)l. Total pressure (including inerts) = 152.0 torr.
Distance YEt3Xn (ton) for ASH, ([on inlet) of:
Down Renclor
=
(cm) PASH~,O 1.5 (3.25 3.0
Find a rate law consistent with the experimental data.
P5-TB The following data were reported [C. N. Hinshelwooc! and P. J. Ackeq, Rroc.
R. Soc. (Lond)., A11.5, 215 (1927)l for a gas-p! ase constant-volume decompo-
sition of dimethyl ether at 504°C in a batch reactor. Initially, only (CH,),O
was present.
:
Erne (s)
,,
(a) Why do you think the total pressure measurement at t = 0 is missing?
Can you estimate it?
(b) Assuming that the reaction
is irreversible and goes to completion, determine the reaction order and
specific reaction rate k.