Page 303 - Elements of Chemical Reaction Engineering 3rd Edition
P. 303
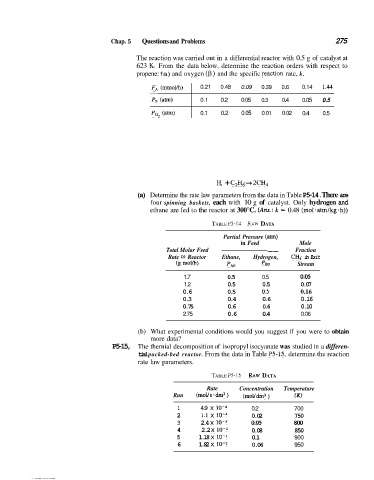
Chap. 5 Questions and Problems 275
The reaction was carried out in a differential reactor with 0.5 g of catalyst at
623 IS. From the data below, determine the reaction orders with respect to
propene: fa) and oxygen (p) and the specific Feaction rate, k.
F!, (mmol/h)
- 0.21 0.48 0.09 0.39 0.6 0.14 1.4t -
P, (am) 0.1 0.2 0.05 0.3 0.4 0.05 0.5
Po, (atm) 0.1 0.2 0.05 0.01 0.02 0.4 0.5
H, + C,H, + 2CH,
(a) Determine the rate law parameters from the data in Table P5-14. There are
four spinning baskets, each with 10 g of catalyst. Only hydrogen and
ethane are fed to the reactor at 300°C. (Arts.: k = 0.48 (mol.atm/kg.h))
TABLE P5-14 RAW DATA
Partial Pressure (am)
in Feed Mole
Total Molar Feed - Fraction
Rate fo Reactor Ethane, Hydrogen, CH, in Exit
(g morn) pBO Stream
1.7 0.5 0.5 0.05
1.2 0.5 0.5 0.07
0.6 0.5 0.5 0.16
0.3 0.4 0.6 0.16
0.75 0.6 0.6 0.10
2.75 0.6 0.4 0.06
(b) What experimental conditions would you suggest if you were to obtain
more data?
P5-15, The thernial decomposition of isopropyl isocyanate was studied in a differen-
tial packed-bed reactor. From the data in Table P5-15, determine the reaction
rate law parameters.
TABLE P5-15 RAW DATA
Rate Concentration Temperature
Run (mol/s.dm3) (moVdm3 ) IKI
1 4.9 x 10-4 0.2 700
2 1.1 x 10-4 0.02 750
3 2.4 x 10-3 0.05 800
4 2.2 x 10-2 0.08 850
5 1.18 X IO-' 0.1 900
6 1.82 x 10-2 0.06 950