Page 307 - Elements of Chemical Reaction Engineering 3rd Edition
P. 307
Chap. 5 Journal Critique Problems 279
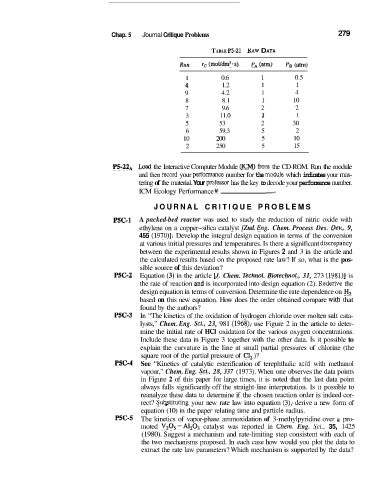
TABLE P5-21 RAW DATA
Run rc (mol/dm3*s) PA (am) P, (atm)
1 0.6 1 0.5
4 1.2 1 1
9 4.2 1 4
8 8.1 1 10
7 9.6 2 2
3 11.0 3 1
5 53 2 30
6 59.3 5 2
10 200 5 10
2 250 5 15
P5-22* bad the Interactive Computer Module froln the CD-ROM. Run the module
and then mrd your perfonnance number for the dule which indicates your mas-
tering of the material. Your professor has the key to decode your performance number.
ICM Ecology Performance # -*
JOURNAL CRITIQUE PROBLEMS
P5C-1 A packed-bed reactor was used to study the reduction of nitric oxide with
ethylene on a copper-silica catalyst [Znd. Eng. Chem. Process Des. Dev., 9,
455 (1970)l. Develop the integral design equation in terms of the conversion
at various initial pressures and temperatures. Is there a significant discre:pancy
between the experimental results shown in Figures 2 and 3 in the article and
the calculated results based on the proposed rate law? If so, what is the pos-
sible source of this deviation?
P5C-2; Equation (3) in the article [J. Chem. Technol. BiotechnoE., 31, 273 (19131)l is
the rate of reaction and is incorporated into design equation (2). Rederive the
design equation in terms of conversion. Determine the rate dependence on H,
based on this new equation. How does the order obtained compare with that
found by the authors?
P5C-3 In “The kinetics of the oxidation of hydrogen chloride over molten salt cata-
lysts,” Chem. Eng. Sci., 23, 981 (1968), use Figure 2 in the article to deter-
mine the initial rate of HCl oxidation for the various oxygen concentrations.
Include these data in Figure 3 together with the other data. Is it possible to
explain the curvature in the line at small partial pressures of chlorine (the
square root of the partial pressure of C1, )?
P5C-4 See “Kinetics of catalytic esterification of terephthalic aci‘d with methanol
vapour,” Chem. Eng. Sci., 28, 337 (1973). When one observes the data points
in Figure 2 of this paper for large times, it is noted that the last data point
always falls significantly off the straight-line interpretation. Is it possible to
reanalyze these data to determine if the chosen reaction order is indeed cor-
rect? Sotptituting your new rate law into equation (3), derive a new form of
equation (10) in the paper relating time and particle radius.
P5C-5 The kinetics of vapor-phase ammoxidation of 3-methylpyridine over <a pro-
moted V20,-A1,0, catalyst was reported in Chem. Eng. Sci., 35, 1425
(1980). Suggest a mechanism and rate-limiting step consistent with each of
the two mechanisms proposed. In each case how would you plot the data to
extract the rate law parameters? Which mechanism is supported by the data?