Page 306 - Elements of Chemical Reaction Engineering 3rd Edition
P. 306
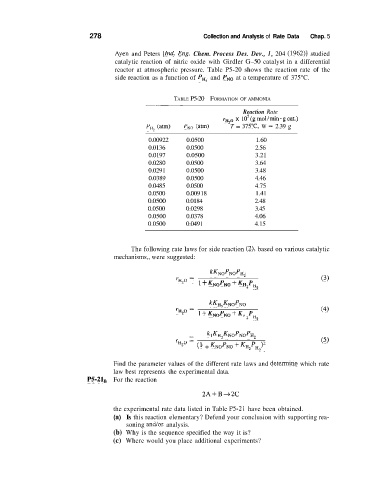
278 Collection and Analysis of Rate Data Chap. 5
Ayen and Peters [Ind. Eng. Chem. Process Des. Dev., 1, 204 (1962)l studied
catalytic reaction of nitric oxide with Girdler G-50 catalyst in a differential
reactor at atmospheric pressure. Table P5-20 shows the reaction rate of the
side reaction as a function of PH, and PNo at a temperature of 375°C.
TABLE P5-20 FORMATION OF AMMONIA
-~
~
Reaction Rate
rHz0 x 1o5(gmoi/min~gcat.~
PH2 (atm) PNo (am) T = 315"C, W = 2.39 g
0.00922 0.0500 1.60
0.0136 0.0500 2.56
0.0197 0.0500 3.21
0.0280 0.0500 3.64
0.029 1 0.0500 3.48
0.0389 0.0500 4.46
0.0485 0.0500 4.75
0.0500 0.009 18 1.41
0.0500 0.0184 2.48
0.0500 0.0298 3.45
0.0500 0.0378 4.06
0.0500 0.0491 4.15
The following rate laws for side reaction (2), based on various catalytic
mechanisms,, were suggested:
~KNOPNOPH~
1 + KNoPNo + K, P (3)
2 H2
kKH,KNOPNO
K,
"2' = 1 $. KNoPN0 i- P (4)
2 H2
- kl KH2KNOPNOPH2
- (5)
(1 + KNop~o + Kn2PH2I2
Find the parameter values of the different rate laws and determiqe which rate
law best represents the experimental data.
P5-21B For the reaction
2A+B+2C
the experimental rate data listed in Table PS-21 have been obtained.
(a) Is this reaction elementary? Defend your conclusion with supporting rea-
soning and/or analysis.
(b) Why is the sequence specified the way it is?
(c) Where would you place additional experiments?