Page 305 - Elements of Chemical Reaction Engineering 3rd Edition
P. 305
Chap. 5 Questions and Problems 277
Graphical techniqueequal area differentiation. (Use 0’s to represent
these points on any graphs you make.)
Differentiating a polynomial. (use x for these points)
(b) Determine the reaction order.
(c) Assume a rate law of the form
Integrate the equation for the combined mole balance and rate law and then
use nonlinear least-squares analysis to determine a and k.
(d) Where would you place additional data points?
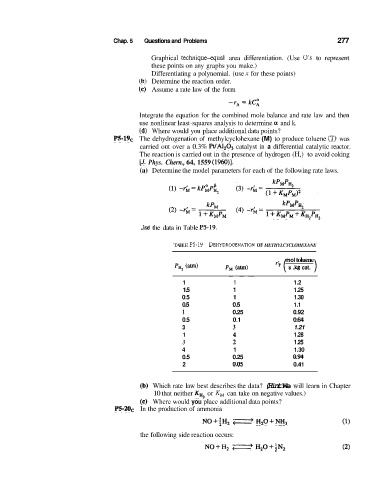
P5-191, The dehydrogenation of methylcyclohexane (M) to produce toluene (T) was
carried out over a 0.3% WA1,03 catalyst in a differential catalytic reactor.
The reaction is carried out in the presence of hydrogen (H,) to avoid coking
[J. Phys. Chern., 64, 1559 (1960)l.
(a) Determine the model parameters for each of the following rate laws.
%e the data in Table P5-19.
TABLE P5-19 DEHYDROGENATION OF METHYLCYCLOHEXANE
mol toluene
‘’ ( s . kg cat. )
~~~
1 1 1.2
1.5 1 1.25
0.5 1 1.30
0.5 0.5 1.1
1 0.25 0.92
0.5 0.1 0.64
3 3 1.21
1 4 1.28
3 2 1.25
4 1 1.30
0.5 0.25 a94
2 0.05 0.41
(b) Which rate law best describes the data? (Hint: We will learn in Chapter
10 that neither KHZ or KM can take on negative values.)
(e) Where would you place additional data points?
P5-2OC In the production of ammonia
N0+iH2 e H,O+NH,
the following side reaction occurs:
NO+H, e H,O+iN,