Page 568 - Elements of Chemical Reaction Engineering Ebook
P. 568
538 Unsteady-State Nonisothermal Reactor Design Chap. 9
Canceling dt, separating variables, integrating, and rearranging (see CD-ROM)
gives
cp(T-To) C @,cpi(T-To) (9-16)
- AH,, ( T)
f
I
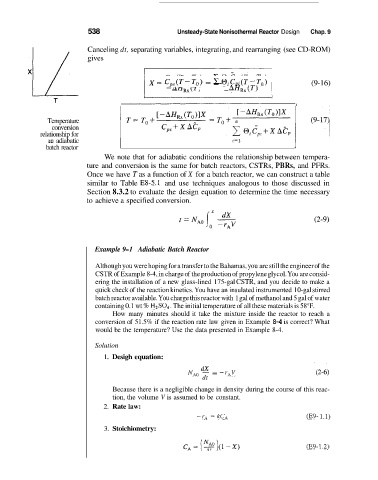
Temperature
conversion
relationship for
an adiabatic l=1 1
batch reactor
We note that for adiabatic conditions the relationship between tempera-
ture and conversion is the same for batch reactors, CSTRs, PBRs, and PFRs.
Once we have T as a function of X for a batch reactor, we can construct a table
similar to Table E8-5.1 and use techniques analogous to those discussed in
Section 8.3.2 to evaluate the design equation to determine the time necessary
to achieve a specified conversion.
Example 9-1 Adiabatic Batch Reactor
Although you were hoping for a transfer to the Bahamas, you are still the engineer of the
CSTR of Example 8-4, in charge of the production of propylene glycol. You are consid-
ering the installation of a new glass-lined 175-gal CSTR, and you decide to make a
quick check of the reaction kinetics. You have an insulated instrumented 10-gal stirred
batch reactor available. You charge this reactor with 1 gal of methanol and 5 gal of water
containing 0.1 wt % H2S04. The initial temperature of all these materials is 58°F.
How many minutes should it take the mixture inside the reactor to reach a
conversion of 51.5% if the reaction rate law given in Example 8-4 is correct? What
would be the temperature? Use the data presented in Example 8-4.
Solution
1. Desigh equation:
dX
N,, - = -r,V
dt
Because there is a negligible change in density during the course of this reac-
tion, the volume V is assumed to be constant.
2. Rate law:
-rA = kCA (E9- 1.1)
3. Stoichiometry:
(E9-1.2)