Page 147 - Academic Press Encyclopedia of Physical Science and Technology 3rd Chemical Engineering
P. 147
P1: LLL Final
Encyclopedia of Physical Science and Technology EN003H-565 June 13, 2001 20:37
Coherent Control of Chemical Reactions 209
Coherent radiation may also be used to control the
external degrees of freedom of a molecule. For example, it
is possible to create a quivering “pendular state” in which
a molecule having an anisotropic polarizability is aligned
along the electric field vector of a laser beam. It is also
possible to use a focused laser beam to deflect a beam of
molecules, perhaps focusing them to a point or steering
them towards a target. Control of external degrees of free-
dom is discussed in Section V. We conclude this article
with a brief discussion of future directions that the field is
likely to take.
II. MODE-SELECTIVE CHEMISTRY
The central concept of mode-selective chemistry is illus-
trated in Fig. 1, which depicts the ground and excited
state potential energy surfaces of a hypothetical triatomic
molecule, ABC. One might wish, for example, to break
selectively the bond between atoms A and B to yield prod-
ucts A+BC. Alternatively, one might wish to activate that
bond so that in a subsequent collision with atom D the
products AD+BC are formed. To achieve either goal it is
necessary to cause bond AB to vibrate, thereby inducing
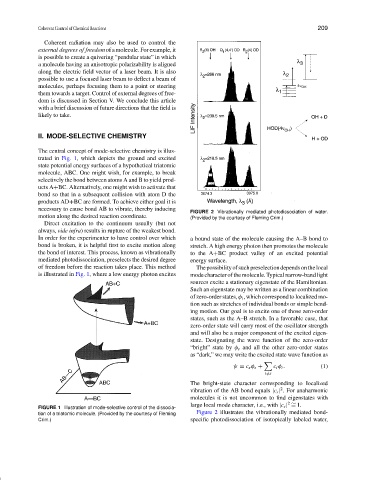
FIGURE 2 Vibrationally mediated photodissociation of water.
motion along the desired reaction coordinate. (Provided by the courtesy of Fleming Crim.)
Direct excitation to the continuum usually (but not
always, vide infra) results in rupture of the weakest bond.
In order for the experimenter to have control over which a bound state of the molecule causing the A–B bond to
bond is broken, it is helpful first to excite motion along stretch. A high energy photon then promotes the molecule
the bond of interest. This process, known as vibrationally to the A+BC product valley of an excited potential
mediated photodissociation, preselects the desired degree energy surface.
of freedom before the reaction takes place. This method Thepossibilityofsuchpreselectiondependsonthelocal
is illustrated in Fig. 1, where a low energy photon excites modecharacterof the molecule. Typical narrow-band light
sources excite a stationary eigenstate of the Hamiltonian.
Such an eigenstate may be written as a linear combination
of zero-order states, φ i , which correspond to localized mo-
tion such as stretches of individual bonds or simple bend-
ing motion. Our goal is to excite one of those zero-order
states, such as the A–B stretch. In a favorable case, that
zero-order state will carry most of the oscillator strength
and will also be a major component of the excited eigen-
state. Designating the wave function of the zero-order
“bright” state by φ s and all the other zero-order states
as “dark,” we may write the excited state wave function as
ψ = c s φ s + c i φ i . (1)
i =s
The bright-state character corresponding to localized
2
vibration of the AB bond equals |c s | . For anaharmonic
molecules it is not uncommon to find eigenstates with
2 ∼
large local mode character, i.e., with |c s | = 1.
FIGURE 1 Illustration of mode-selective control of the dissocia-
tion of a triatomic molecule. (Provided by the courtesy of Fleming Figure 2 illustrates the vibrationally mediated bond-
Crim.) specific photodissociation of isotopically labeled water,