Page 367 - Academic Press Encyclopedia of Physical Science and Technology 3rd Chemical Engineering
P. 367
P1: GLQ Final Pages
Encyclopedia of Physical Science and Technology EN009K-419 July 19, 2001 20:57
302 Membranes, Synthetic, Applications
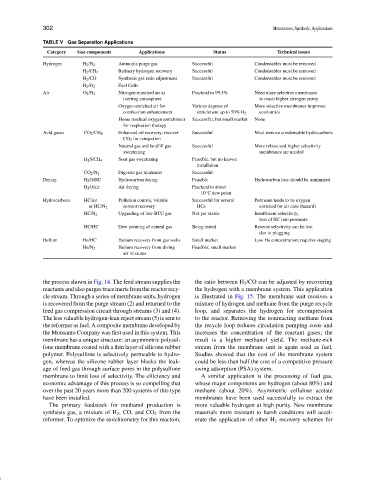
TABLE V Gas Separation Applications
Category Gas components Applications Status Technical issues
Hydrogen H 2 /N 2 Ammonia purge gas Successful Condensables must be removed
H 2 /CH 4 Refinery hydrogen recovery Successful Condensables must be removed
H 2 /CO Synthesis gas ratio adjustment Successful Condensables must be removed
H 2 /O 2 Fuel Cells
Air O 2 /N 2 Nitrogen-enriched air as Practical to 99.5% Need more selective membranes
inerting atmosphere to reach higher nitrogen purity
Oxygen-enriched air for Various degrees of More selective membranes improves
combustion enhancement enrichment up to 50% O 2 economics
Home medical oxygen enrichment Successful, but small market None
for respiration therapy
Acid gases CO 2 /CH 4 Enhanced oil recovery; recover Successful Must remove condensable hydrocarbons
CO 2 for reinjection
Natural gas and landfill gas Successful More robust and higher selectivity
sweetening membranes are needed
H 2 S/CH 4 Sour gas sweetening Feasible, but no known
installation
CO 2 /N 2 Digester gas treatment Successful
Drying H 2 O/HC Hydrocarbon drying Feasible Hydrocarbon loss should be minimized
H 2 O/air Air drying Practical to about
◦
−10 C dew point
Hydrocarbons HC/air Pollution control, volatile Successful for several Permeate tends to be oxygen
or HC/N 2 solvent recovery HCs enriched for air case (hazard)
HC/N 2 Upgrading of low-BTU gas Not yet viable Insufficient selectivity;
loss of HC into permeate
HC/HC Dew pointing of natural gas Being tested Reverse selectivity can be lost
due to plugging
Helium He/HC Helium recovery from gas wells Small market Low He concentration; requires staging
He/N 2 Helium recovery from diving Feasible; small market
air mixtures
the process shown in Fig. 14. The feed stream supplies the the ratio between H 2 /CO can be adjusted by recovering
reactants and also purges trace inerts from the reactor recy- the hydrogen with a membrane system. This application
cle stream. Through a series of membrane units, hydrogen is illustrated in Fig. 15. The membrane unit receives a
is recovered from the purge stream (2) and returned to the mixture of hydrogen and methane from the purge recycle
feed gas compression circuit through streams (3) and (4). loop, and separates the hydrogen for recompression
The less valuable hydrogen-lean reject stream (5) is sent to to the reactor. Removing the nonreacting methane from
the reformer as fuel. A composite membrane developed by the recycle loop reduces circulation pumping costs and
the Monsanto Company was first used in this system. This increases the concentration of the reactant gases; the
membrane has a unique structure: an asymmetric polysul- result is a higher methanol yield. The methane-rich
fone membrane coated with a thin layer of silicone rubber stream from the membrane unit is again used as fuel.
polymer. Polysulfone is selectively permeable to hydro- Studies showed that the cost of the membrane system
gen, whereas the silicone rubber layer blocks the leak- could be less than half the cost of a competitive pressure
age of feed gas through surface pores in the polysulfone swing adsorption (PSA) system.
membrane to limit loss of selectivity. The efficiency and A similar application is the processing of fuel gas,
economic advantage of this process is so compelling that whose major components are hydrogen (about 80%) and
over the past 20 years more than 200 systems of this type methane (about 20%). Asymmetric cellulose acetate
have been installed. membranes have been used successfully to extract the
The primary feedstock for methanol production is more valuable hydrogen at high purity. New membrane
synthesis gas, a mixture of H 2 , CO, and CO 2 from the materials more resistant to harsh conditions will accel-
reformer. To optimize the stoichiometry for this reaction, erate the application of other H 2 recovery schemes for