Page 8 - Academic Press Encyclopedia of Physical Science and Technology 3rd Chemical Engineering
P. 8
P1: LDK Revised Pages
Encyclopedia of Physical Science and Technology EN001H-01 May 7, 2001 16:18
Absorption (Chemical Engineering) 5
Henry’s law is usually a reasonable approximation at low
and moderate concentrations, at constant temperature, and
at relatively low pressures (generally less than 5 atm; how-
ever, the law may be obeyed at higher pressure at low
solubilities).
If a gas mixture containing several components is in
equilibrium with a liquid, Henry’s law applies separately
so long as the liquid is dilute in all the components. If a
component is almost insoluble in the liquid, for example,
air in water, it has a very high Henry’s law constant and a
high value of m in Eq. (1). Such a component is absorbed
in negligible quantities or by the liquid, and it is often
referred to as an inert component. The nature and type of
the inert component have little effect on the equilibrium
curve.
Equilibrium data for absorption are usually available in
the literature in three forms:
1. Solubility data, expressed either as mole percent,
mass percent, or Henry’s law constants
2. Pure-component vapor pressure data
3. Equilibrium distribution coefficients (K values)
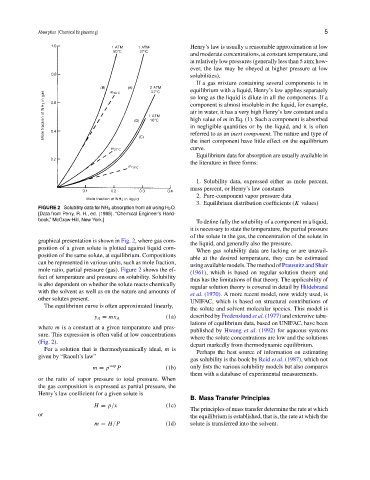
FIGURE 2 Solubility data for NH 3 absorption from air using H 2 O.
[Data from Perry, R. H., ed. (1985). “Chemical Engineer’s Hand-
book,” McGraw-Hill, New York.]
To define fully the solubility of a component in a liquid,
it is necessary to state the temperature, the partial pressure
of the solute in the gas, the concentration of the solute in
graphical presentation is shown in Fig. 2, where gas com-
the liquid, and generally also the pressure.
position of a given solute is plotted against liquid com-
When gas solubility data are lacking or are unavail-
position of the same solute, at equilibrium. Compositions
able at the desired temperature, they can be estimated
can be represented in various units, such as mole fraction,
using available models. The method of Prausnitz and Shair
mole ratio, partial pressure (gas). Figure 2 shows the ef-
(1961), which is based on regular solution theory and
fect of temperature and pressure on solubility. Solubility
thus has the limitations of that theory. The applicability of
is also dependent on whether the solute reacts chemically
regular solution theory is covered in detail by Hildebrand
with the solvent as well as on the nature and amounts of
et al. (1970). A more recent model, now widely used, is
other solutes present.
UNIFAC, which is based on structural contributions of
The equilibrium curve is often approximated linearly,
the solute and solvent molecular species. This model is
y A = mx A (1a) described by Fredenslund et al. (1977) and extensive tabu-
lations of equilibrium data, based on UNIFAC, have been
where m is a constant at a given temperature and pres-
published by Hwang et al. (1992) for aqueous systems
sure. This expression is often valid at low concentrations
where the solute concentrations are low and the solutions
(Fig. 2).
depart markedly from thermodynamic equilibrium.
For a solution that is thermodynamically ideal, m is
Perhaps the best source of information on estimating
given by “Raoult’slaw”
gas solubility is the book by Reid et al. (1987), which not
m = p vap P (1b) only lists the various solubility models but also compares
them with a database of experimental measurements.
or the ratio of vapor pressure to total pressure. When
the gas composition is expressed as partial pressure, the
Henry’s law coefficient for a given solute is
B. Mass Transfer Principles
H = p/x (1c)
The principles of mass transfer determine the rate at which
or the equilibrium is established, that is, the rate at which the
m = H/P (1d) solute is transferred into the solvent.