Page 12 - Academic Press Encyclopedia of Physical Science and Technology 3rd Chemical Engineering
P. 12
P1: LDK Revised Pages
Encyclopedia of Physical Science and Technology EN001H-01 May 7, 2001 16:18
Absorption (Chemical Engineering) 9
transfer behavior with the aid of material and heat bal-
ances. In order to apply these balances, the equipment
must be described in terms of a mathematical model.
In this section, the equations are presented for the com-
mon types of contactors: differential contactors and stage-
wise contactors. The equations are developed for the case
of steady-state, countercurrent contacting of liquid and gas
with negligible heat effects, with a single-component ab-
sorption. Some discussion of extensions to other situations
follows.
A. Differential Contactors
1. Material Balances
Differential contactors include packed towers, spray tow-
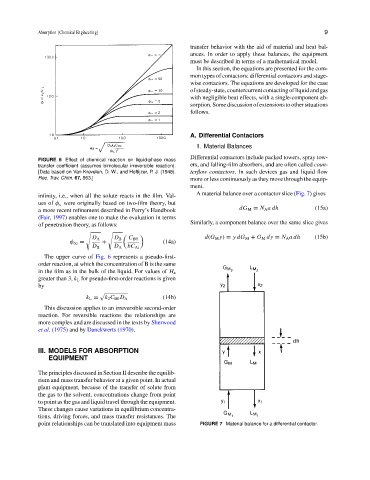
FIGURE 6 Effect of chemical reaction on liquid-phase mass
transfer coefficient (assumes bimolecular irreversible reaction). ers, and falling-film absorbers, and are often called coun-
[Data based on Van Krevelen, D. W., and Hoftijzer, P. J. (1948). terflow contactors. In such devices gas and liquid flow
Rec. Trav. Chim. 67, 563.] more or less continuously as they move through the equip-
ment.
infinity, i.e., when all the solute reacts in the film. Val- A material balance over a contactor slice (Fig. 7) gives
ues of φ x were originally based on two-film theory, but
dG M = N A adh (15a)
a more recent refinement described in Perry’s Handbook
(Fair, 1997) enables one to make the evaluation in terms
Similarly, a component balance over the same slice gives
of penetration theory, as follows:
d(G M y) = ydG M + G M dy = N A adh (15b)
D A D B C B0
φ ∞ = + (14a)
D B D A bC Ai
The upper curve of Fig. 6 represents a pseudo-first-
order reaction, at which the concentration of B is the same
in the film as in the bulk of the liquid. For values of H a
greater than 3, k L for pseudo-first-order reactions is given
by
k L = k 2 C B0 D A (14b)
This discussion applies to an irreversible second-order
reaction. For reversible reactions the relationships are
more complex and are discussed in the texts by Sherwood
et al. (1975) and by Danckwerts (1970).
III. MODELS FOR ABSORPTION
EQUIPMENT
The principles discussed in Section II describe the equilib-
rium and mass transfer behavior at a given point. In actual
plant equipment, because of the transfer of solute from
the gas to the solvent, concentrations change from point
to point as the gas and liquid travel through the equipment.
These changes cause variations in equilibrium concentra-
tions, driving forces, and mass transfer resistances. The
point relationships can be translated into equipment mass FIGURE 7 Material balance for a differential contactor.