Page 16 - Academic Press Encyclopedia of Physical Science and Technology 3rd Chemical Engineering
P. 16
P1: LDK Revised Pages
Encyclopedia of Physical Science and Technology EN001H-01 May 7, 2001 16:18
Absorption (Chemical Engineering) 13
This absorption factor is the ratio of slope of the operating
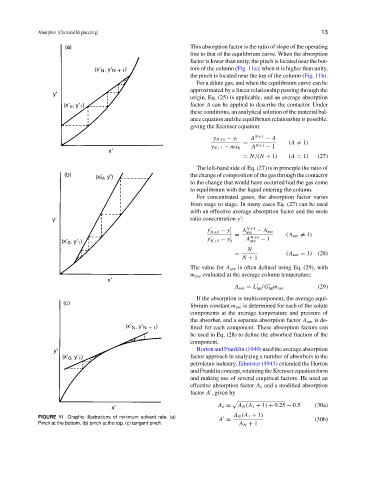
line to that of the equilibrium curve. When the absorption
factor is lower than unity, the pinch is located near the bot-
tom of the column (Fig. 11a); when it is higher than unity,
the pinch is located near the top of the column (Fig. 11b).
For a dilute gas, and when the equilibrium curve can be
approximated by a linear relationship passing through the
origin, Eq. (25) is applicable, and an average absorption
factor A can be applied to describe the contactor. Under
these conditions, an analytical solution of the material bal-
ance equation and the equilibrium relationship is possible,
giving the Kremser equation:
A N+1 − A
y N+1 − y 1
= N+1 (A = 1)
y N+1 − mx 0 A − 1
= N/(N + 1) (A = 1) (27)
The left-hand side of Eq. (27) is in principle the ratio of
the change of composition of the gas through the contactor
to the change that would have occurred had the gas come
to equilibrium with the liquid entering the column.
For concentrated gases, the absorption factor varies
from stage to stage. In many cases Eq. (27) can be used
with an effective average absorption factor and the mole
ratio concentration y :
y N+1 − y 1 A N+1 − A ave
ave
= (A ave = 1)
y − y A N+1 − 1
N+1 0 ave
N
= (A ave = 1) (28)
N + 1
The value for A ave is often defined using Eq. (29), with
m ave evaluated at the average column temperature:
A ave = L /G m ave (29)
M M
If the absorption is multicomponent, the average equi-
librium constant m ave is determined for each of the solute
components at the average temperature and pressure of
the absorber, and a separate absorption factor A ave is de-
fined for each component. These absorption factors can
be used in Eq. (28) to define the absorbed fraction of the
component.
Horton and Franklin (1940) used the average absorption
factor approach in analyzing a number of absorbers in the
petroleum industry. Edmister (1943) extended the Horton
andFranklinconcept,retainingtheKremserequationform
and making use of several empirical factors. He used an
effective absorption factor A e and a modified absorption
factor A , given by
A e = A N (A 1 + 1) + 0.25 − 0.5 (30a)
A N (A 1 + 1)
FIGURE 11 Graphic illustrations of minimum solvent rate. (a)
A = (30b)
Pinch at the bottom, (b) pinch at the top, (c) tangent pinch. A N + 1