Page 10 - Academic Press Encyclopedia of Physical Science and Technology 3rd Chemical Engineering
P. 10
P1: LDK Revised Pages
Encyclopedia of Physical Science and Technology EN001H-01 May 7, 2001 16:18
Absorption (Chemical Engineering) 7
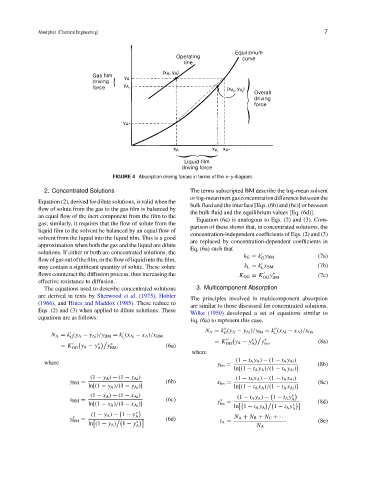
FIGURE 4 Absorption driving forces in terms of the x–y diagram.
2. Concentrated Solutions The terms subscripted BM describe the log-mean solvent
orlog-meaninertgasconcentrationdifferencebetweenthe
Equation (2), derived for dilute solutions, is valid when the
bulk fluid and the interface [Eqs. (6b) and (6c)] or between
flow of solute from the gas to the gas film is balanced by
the bulk fluid and the equilibrium values [Eq. (6d)].
an equal flow of the inert component from the film to the
Equation (6a) is analogous to Eqs. (2) and (3). Com-
gas; similarly, it requires that the flow of solute from the
parison of these shows that, in concentrated solutions, the
liquid film to the solvent be balanced by an equal flow of
concentration-independent coefficients of Eqs. (2) and (3)
solvent from the liquid into the liquid film. This is a good
are replaced by concentration-dependent coefficients in
approximation when both the gas and the liquid are dilute
Eq. (6a) such that
solutions. If either or both are concentrated solutions, the
k G = k y BM (7a)
G
flowofgasoutofthefilm,ortheflowofliquidintothefilm,
(7b)
may contain a significant quantity of solute. These solute k L = k x BM
L
flows counteract the diffusion process, thus increasing the K OG = K y ∗ (7c)
OG BM
effective resistance to diffusion.
The equations used to describe concentrated solutions 3. Multicomponent Absorption
are derived in texts by Sherwood et al. (1975), Hobler
The principles involved in multicomponent absorption
(1966), and Hines and Maddox (1985). These reduce to
are similar to those discussed for concentrated solutions.
Eqs. (2) and (3) when applied to dilute solutions. These
Wilke (1950) developed a set of equations similar to
equations are as follows:
Eq. (6a) to represent this case,
N A = k (y A − y Ai )/y fm = k (x Ai − x A )/x fm
L
G
N A = k (y A − y Ai )/y BM = k (x Ai − x A )/x BM
L
G
∗
= K OG y A − y A ∗ y , (8a)
fm
= K OG y A − y A ∗ y ∗ BM , (6a)
where
(1 − t A y A ) − (1 − t A y Ai )
where y fm = (8b)
ln[(1 − t A y A )/(1 − t A y Ai )]
(1 − y A ) − (1 − y Ai ) (1 − t A x A ) − (1 − t A x Ai )
y BM = (6b) x fm = (8c)
ln[(1 − y A )/(1 − y Ai )] ln[(1 − t A x A )/(1 − t A x Ai )]
(1 − x A ) − (1 − x Ai ) (1 − t A y A ) − 1 − t A y ∗
x BM = (6c) y ∗ = A (8d)
ln[(1 − x A )/(1 − x Ai )] fm ∗
ln 1 − t A y A 1 − t A y
A
(1 − y A ) − 1 − y A ∗
y ∗ = (6d) N A + N B + N C + ···
BM t A = (8e)
ln (1 − y A ) 1 − y ∗
A N A