Page 216 - Academic Press Encyclopedia of Physical Science and Technology 3rd Organic Chemistry
P. 216
P1: GPJ/GLT P2: GQT Final Pages
Encyclopedia of Physical Science and Technology en011-542 July 26, 2001 15:33
502 Organic Chemistry, Synthesis
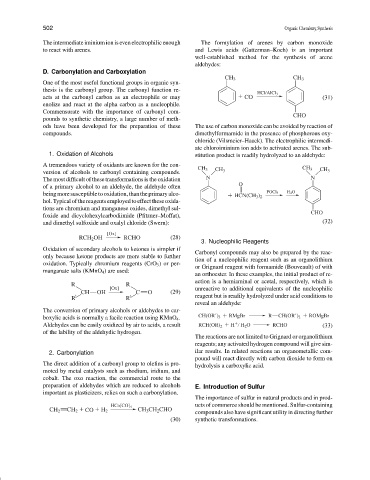
The intermediate iminium ion is even electrophilic enough The formylation of arenes by carbon monoxide
to react with arenes. and Lewis acids (Gatterman–Koch) is an important
well-established method for the synthesis of arene
aldehydes:
D. Carbonylation and Carboxylation
CH 3 CH 3
One of the most useful functional groups in organic syn-
thesis is the carbonyl group. The carbonyl function re-
HCl/AlCl 3
acts at the carbonyl carbon as an electrophile or may CO (31)
enolize and react at the alpha carbon as a nucleophile.
Commensurate with the importance of carbonyl com-
CHO
pounds to synthetic chemistry, a large number of meth-
ods have been developed for the preparation of these The use of carbon monoxide can be avoided by reaction of
compounds. dimethylformamide in the presence of phosphorous oxy-
chloride (Vilsmeier–Haack). The electrophilic intermedi-
ate chloroiminium ion adds to activated arenes. The sub-
1. Oxidation of Alcohols stitution product is readily hydrolyzed to an aldehyde:
A tremendous variety of oxidants are known for the con-
CH 3 CH 3 CH 3 CH 3
version of alcohols to carbonyl containing compounds.
The most difficult of these transformations is the oxidation N N
O
of a primary alcohol to an aldehyde, the aldehyde often
beingmoresusceptibletooxidation,thantheprimaryalco- POCl 3 H 2 O
HCN(CH 3 ) 2
hol.Typicalofthereagentsemployedtoeffecttheseoxida-
tions are chromium and manganese oxides, dimethyl sul-
CHO
foxide and dicyclohexylcarbodiimide (Pfitzner–Moffat),
and dimethyl sulfoxide and oxalyl chloride (Swern): (32)
[Ox]
RCH 2 OH RCHO (28)
3. Nucleophilic Reagents
Oxidation of secondary alcohols to ketones is simpler if
Carbonyl compounds may also be prepared by the reac-
only because ketone products are more stable to further
tion of a nucleophilic reagent such as an organolithium
oxidation. Typically chromium reagents (CrO 3 ) or per-
or Grignard reagent with formamide (Bouveault) of with
manganate salts (KMnO 4 ) are used:
an orthoester. In these examples, the initial product of re-
action is a hemiaminal or acetal, respectively, which is
R R
[Ox] unreactive to additional equivalents of the nucleophilic
CH OH C O (29)
R R reagent but is readily hydrolyzed under acid conditions to
reveal an aldehyde:
The conversion of primary alcohols or aldehydes to car-
boxylic acids is normally a facile reaction using KMnO 4 . CH(OR ) 3 RMgBr R CH(OR ) 2 ROMgBr
Aldehydes can be easily oxidized by air to acids, a result RCH(OR) 2 H H 2 O RCHO (33)
of the lability of the aldehydic hydrogen.
The reactions are not limited to Grignard or organolithium
reagents; any activated hydrogen compound will give sim-
2. Carbonylation ilar results. In related reactions an organometallic com-
pound will react directly with carbon dioxide to form on
The direct addition of a carbonyl group to olefins is pro- hydrolysis a carboxylic acid.
moted by metal catalysts such as rhodium, iridium, and
cobalt. The oxo reaction, the commercial route to the
preparation of aldehydes which are reduced to alcohols E. Introduction of Sulfur
important as plasticizers, relies on such a carbonylation.
The importance of sulfur in natural products and in prod-
ucts of commerce should be mentioned. Sulfur-containing
HCo[CO] 4
CH 2 CH 2 CO H 2 CH 3 CH 2 CHO
compounds also have significant utility in directing further
(30) synthetic transformations.