Page 219 - Academic Press Encyclopedia of Physical Science and Technology 3rd Organic Chemistry
P. 219
P1: GPJ/GLT P2: GQT Final Pages
Encyclopedia of Physical Science and Technology en011-542 July 26, 2001 15:33
Organic Chemistry, Synthesis 505
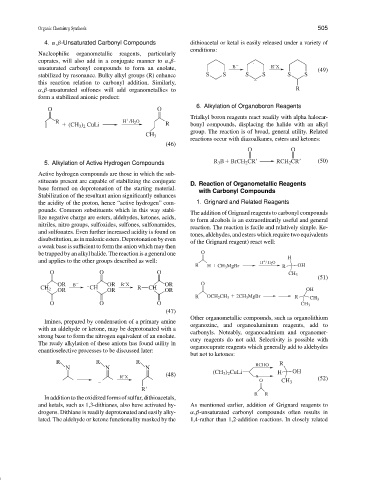
4. α,β-Unsaturated Carbonyl Compounds dithioacetal or ketal is easily released under a variety of
conditions:
Nucleophilic organometallic reagents, particularly
cuprates, will also add in a conjugate manner to α,β- .
unsaturated carbonyl compounds to form an enolate, B R X (49)
stabilized by resonance. Bulky alkyl groups (R) enhance S S S S S S
this reaction relation to carbonyl addition. Similarly,
α,β-unsaturated sulfones will add organometallics to R
form a stabilized anionic product:
6. Alkylation of Organoboron Reagents
O O
Trialkyl boron reagents react readily with alpha halocar-
R H /H 2 O
(CH 3 ) 2 CuLi R bonyl compounds, displacing the halide with an alkyl
group. The reaction is of broad, general utility. Related
CH 3
reactions occur with diazoalkanes, esters and ketones:
(46)
O O
5. Alkylation of Active Hydrogen Compounds R 3 B BrCH 2 CR RCH 2 CR (50)
Active hydrogen compounds are those in which the sub-
stituents present are capable of stabilizing the conjugate
D. Reaction of Organometallic Reagents
base formed on deprotonation of the starting material.
with Carbonyl Compounds
Stabilization of the resultant anion significantly enhances
the acidity of the proton, hence “active hydrogen” com- 1. Grignard and Related Reagents
pounds. Common substituents which in this way stabi-
The addition of Grignard reagents to carbonyl compounds
lize negative charge are esters, aldehydes, ketones, acids,
to form alcohols is an extraordinarily useful and general
nitriles, nitro groups, sulfoxides, sulfones, sulfonamides,
reaction. The reaction is facile and relatively simple. Ke-
and sulfonates. Even further increased acidity is found on
tones, aldehydes, and esters which require two equivalents
disubstitution, as in malonic esters. Deprotonation by even
of the Grignard reagent) react well:
a weak base is sufficient to form the anion which may then
be trapped by an alkyl halide. The reaction is a general one O
H
and applies to the other groups described as well: H / H 2 O
R H CH 3 MgBr R OH
O O O
CH 3
(51)
OR B OR R X OR O
CH 2 CH R CH OH
OR OR OR
R OCH 2 CH 3 2CH 3 MgBr R
CH 3
O O O CH 3
(47)
Other organometallic compounds, such as organolithium
Imines, prepared by condensation of a primary amine
organozinc, and organoaluminum reagents, add to
with an aldehyde or ketone, may be deprotonated with a
carbonyls. Noteably, organocadmium and organomer-
strong base to form the nitrogen equivalent of an enolate.
cury reagents do not add. Selectivity is possible with
The ready alkylation of these anions has found utility in organocuprate reagents which generally add to aldehydes
enantioselective processes to be discussed later:
but not to ketones:
R R R RCHO R
N N N
(48) (CH 3 ) 2 CuLi H OH
R X x (52)
O
CH 3
R
R R
In addition to the oxidized forms of sulfur, dithioacetals,
and ketals, such as 1,3-dithianes, also have activated hy- As mentioned earlier, addition of Grignard reagents to
drogens. Dithiane is readily deprotonated and easily alky- α,β-unsaturated carbonyl compounds often results in
lated. The aldehyde or ketone functionality masked by the 1,4-rather than 1,2-addition reactions. In closely related