Page 222 - Academic Press Encyclopedia of Physical Science and Technology 3rd Organic Chemistry
P. 222
P1: GPJ/GLT P2: GQT Final Pages
Encyclopedia of Physical Science and Technology en011-542 July 26, 2001 15:33
508 Organic Chemistry, Synthesis
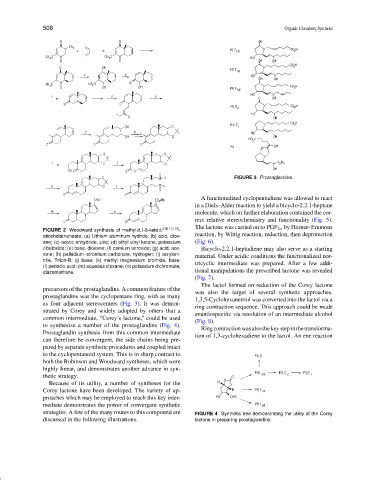
FIGURE 3 Prostaglandins.
A functionalized cyclopentadiene was allowed to react
in a Diels–Alder reaction to yield a bicyclo-2.2.1-heptane
molecule, which on further elaboration contained the cor-
rect relative stereochemistry and functionality (Fig. 5).
The lactone was carried on to PGF 2α by Horner–Emmons
FIGURE 2 Woodward synthesis of methyl d ,l-3-keto 4.9(11).16 -
etiocholatrienoate; (a) Lithium aluminum hydride; (b) acid, diox- reaction, by Wittig reaction, reduction, then deprotection
ane; (c) acetic anhydride, zinc; (d) ethyl vinyl ketone, potassium (Fig. 6).
t-butoxide; (e) base, dioxane; (f) osmium tetroxide; (g) acid, ace- Bicyclo-2.2.1-heptadiene may also serve as a starting
tone; (h) palladium–strontium carbonate, hydrogen; (i) acryloni- material. Under acidic conditions the functionalized nor-
trile, Triton-B; (j) base; (k) methyl magnesium bromide, base;
(l) periodic acid; (m) aqueous dioxane; (n) potassium dichromate, tricyclic intermediate was prepared. After a few addi-
diazomethane. tional manipulations the prescribed lactone was revealed
(Fig. 7).
The lactol formed on reduction of the Corey lactone
precursors of the prostaglandins. A common feature of the
was also the target of several synthetic approaches.
prostaglandins was the cyclopentane ring, with as many
1,3,5-Cyclohexanetriol was converted into the lactol via a
as four adjacent stereocenters (Fig. 3). It was demon-
ring contraction sequence. This approach could be made
strated by Corey and widely adopted by others that a
enantiospecific via resolution of an intermediate alcohol
common intermediate, “Corey’s lactone,” could be used
(Fig. 8).
to synthesize a number of the prostaglandins (Fig. 4).
Ringcontractionwasalsothekeystepinthetransforma-
Prostaglandin synthesis from this common intermediate
tion of 1,3-cyclohexadiene to the lactol. An ene reaction
can therefore be convergent, the side chains being pre-
pared by separate synthetic procedures and coupled intact
to the cyclopentanoid system. This is in sharp contrast to
both the Robinson and Woodward syntheses, which were
highly linear, and demonstrates another advance in syn-
thetic strategy.
Because of its utility, a number of syntheses for the
Corey lactone have been developed. The variety of ap-
proaches which may be employed to reach this key inter-
mediate demonstrates the power of convergent synthetic
strategies. A few of the many routes to this compound are FIGURE 4 Synthetic tree demonstrating the utility of the Corey
discussed in the following illustrations. lactone in preparing prostaglandins.