Page 224 - Academic Press Encyclopedia of Physical Science and Technology 3rd Organic Chemistry
P. 224
P1: GPJ/GLT P2: GQT Final Pages
Encyclopedia of Physical Science and Technology en011-542 July 26, 2001 15:33
510 Organic Chemistry, Synthesis
pheyl borane, prepared by reaction of two equivalents of
α-pinene with borane:
) 2 BH
(62)
BH 3
Reaction of the diisopinocampheyl borane with 2-methyl-
1-alkenes,leadsonoxidationtoadisappointing21%enan-
tiomeric excess (i.e., the excess of one enantiomer relative
to the other):
BH B
) 2 ) 2
H
HO
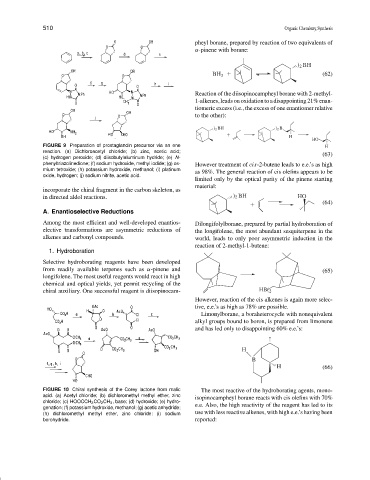
FIGURE 9 Preparation of prostaglandin precursor via an ene H
reaction. (a) Dichloroacetyl chloride; (b) zinc, acetic acid;
(63)
(c) hydrogen peroxide; (d) diisobutylaluminum hydride; (e) N-
phenyltriazolinedione; (f) sodium hydroxide, methyl iodide; (g) os- However treatment of cis-2-butene leads to e.e.’s as high
mium tetroxide; (h) potassium hydroxide, methanol; (i) platinum as 98%. The general reaction of cis olefins appears to be
oxide, hydrogen; (j) sodium nitrite, acetic acid.
limited only by the optical purity of the pinene starting
material:
incorporate the chiral fragment in the carbon skeleton, as
in directed aldol reactions. ) 2 BH HO
(64)
A. Enantioselective Reductions
Among the most efficient and well-developed enantios- Dilongifolylborane, prepared by partial hydroboration of
elective transformations are asymmetric reductions of the longifolene, the most abundant sesquiterpene in the
alkenes and carbonyl compounds. world, leads to only poor asymmetric induction in the
reaction of 2-methyl-1-butene:
1. Hydroboration
Selective hydroborating reagents have been developed
from readily available terpenes such as α-pinene and (65)
longifolene. The most useful reagents would react in high
chemical and optical yields, yet permit recycling of the
chiral auxiliary. One successful reagent is diisopinocam- HB( 2
However, reaction of the cis alkenes is again more selec-
tive, e.e.’s as high as 78% are possible.
Limonylborane, a boraheterocycle with nonequivalent
alkyl groups bound to boron, is prepared from limonene
and has led only to disappointing 60% e.e.’s:
H
B
H (66)
FIGURE 10 Chiral synthesis of the Corey lactone from malic The most reactive of the hydroborating agents, mono-
acid. (a) Acetyl chloride; (b) dichloromethyl methyl ether, zinc isopinocampheyl borane reacts with cis olefins with 70%
chloride; (c) HOOCCH 2 CO 2 CH 3 , base; (d) hydroxide; (e) hydro-
genation; (f) potassium hydroxide, methanol; (g) acetic anhydride; e.e. Also, the high reactivity of the reagent has led to its
(h) dichloromethyl methyl ether, zinc chloride: (i) sodium use with less reactive alkenes, with high e.e.’s having been
borohydride. reported: