Page 227 - Academic Press Encyclopedia of Physical Science and Technology 3rd Organic Chemistry
P. 227
P1: GPJ/GLT P2: GQT Final Pages
Encyclopedia of Physical Science and Technology en011-542 July 26, 2001 15:33
Organic Chemistry, Synthesis 513
S O M A. Biomimetic Polyene Cyclization
(78) The independent proposal of groups at Columbia Uni-
Nu L versity and the ETH in Zurich that the stereoselectivity
R
observed on the cyclization of polyenes is a consequence
More complete analyses, taking into consideration the na- of the olefin stereochemistry constitutes the basis of the
ture of the flanking substituents, which employ orbital Stork–Eschenmoser hypothesis. The epoxide-initiated cy-
overlap arguments, dipole effects, and chelation, have clizationofsqualeneresultsinthestereospecificformation
been advanced to explain results which cannot be under- of dammaradienol. The all-trans double bond geometry of
stood by the simple Cram model. As might be deduced squalene results in the trans–anti-stereochemistry of the
from this discussion, diastereofaceselectivity is remark- ring junctions in dammaradienol:
ably dependent on the nature of the reactants.
Asymmetric induction is also possible in the reaction
of chiral enolates with achiral aldehydes and ketones.
This approach has been successfully employed with chiral
Squalene
imides, amides, and alpha hydroxyketones:
O O O OR O
R R R OSiMe 2 t-Bu (81)
N O N
CH 3 Ph
(79)
HO
The asymmetric inductions realized with these chi-
ral reagents may be amplified remarkably by their reac-
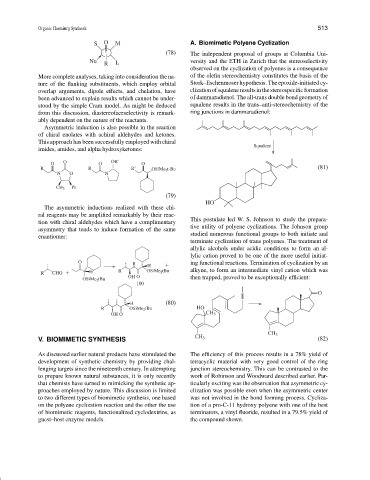
This postulate led W. S. Johnson to study the prepara-
tion with chiral aldehydes which have a complimentary
tive utility of polyene cyclizations. The Johnson group
asymmetry that tends to induce formation of the same
studied numerous functional groups to both initiate and
enantiomer:
terminate cyclization of trans polyenes. The treatment of
allylic alcohols under acidic conditions to form an al-
lylic cation proved to be one of the more useful initiat-
O ing functional reactions. Termination of cyclization by an
H
R CHO H R OSiMe 2 tBu alkyne, to form an intermediate vinyl cation which was
OSiMe 2 tBu OH O then trapped, proved to be exceptionally efficient:
100
O
H (80)
R OSiMe 2 tBu HO
OH O CH 3
CH 3
V. BIOMIMETIC SYNTHESIS CH 3 (82)
As discussed earlier natural products have stimulated the The efficiency of this process results in a 78% yield of
development of synthetic chemistry by providing chal- tetracyclic material with very good control of the ring
lenging targets since the nineteenth century. In attempting junction stereochemistry. This can be contrasted to the
to prepare known natural substances, it is only recently work of Robinson and Woodward described earlier. Par-
that chemists have turned to mimicking the synthetic ap- ticularly exciting was the observation that asymmetric cy-
proaches employed by nature. This discussion is limited clization was possible even when the asymmetric center
to two different types of biomimetic synthesis, one based was not involved in the bond forming process. Cycliza-
on the polyene cyclization reaction and the other the use tion of a pro-C-11 hydroxy polyene with one of the best
of biomimetic reagents, functionalized cyclodextrins, as terminators, a vinyl fluoride, resulted in a 79.5% yield of
guest–host enzyme models. the compound shown.