Page 223 - Academic Press Encyclopedia of Physical Science and Technology 3rd Organic Chemistry
P. 223
P1: GPJ/GLT P2: GQT Final Pages
Encyclopedia of Physical Science and Technology en011-542 July 26, 2001 15:33
Organic Chemistry, Synthesis 509
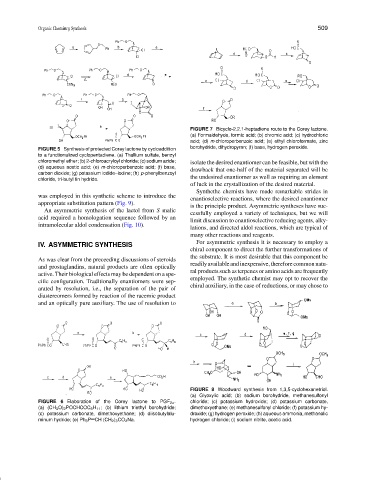
FIGURE 7 Bicyclo-2.2.1-heptadiene route to the Corey lactone.
(a) Formaldehyde, formic acid; (b) chromic acid; (c) hydrochloric
acid; (d) m-chloroperbenzoic acid; (e) ethyl chloroformate, zinc
borohydride, dihydropyran; (f) base, hydrogen peroxide.
FIGURE 5 Synthesis of protected Corey lactone by cycloaddition
to a functionalized cyclopentadiene. (a) Thallium sulfate, benzyl
chloromethyl ether; (b) 2-chloroacryloyl chloride; (c) sodium azide; isolate the desired enantiomer can be feasible, but with the
(d) aqueous acetic acid; (e) m-chloroperbenzoic acid; (f) base,
drawback that one-half of the material separated will be
carbon dioxide; (g) potassium iodide–iodine; (h) p-phenylbenzoyl
chloride, tri-butyl tin hydride. the undesired enantiomer as well as requiring an element
of luck in the crystallization of the desired material.
Synthethc chemists have made remarkable strides in
was employed in this synthetic scheme to introduce the
enantioselective reactions, where the desired enantiomer
appropriate substitution pattern (Fig. 9).
is the principle product. Asymmetric syntheses have suc-
An asymmetric synthesis of the lactol from S malic
cessfully employed a variety of techniques, but we will
acid required a homologation sequence followed by an
limit discussion to enantioselective reducing agents, alky-
intramolecular aldol condensation (Fig. 10).
lations, and directed aldol reactions, which are typical of
many other reactions and reagents.
For asymmetric synthesis it is necessary to employ a
IV. ASYMMETRIC SYNTHESIS
chiral component to direct the further transformations of
the substrate. It is most desirable that this component be
As was clear from the preceeding discussions of steroids
readilyavailableandinexpensive,thereforecommonnatu-
and prostaglandins, natural products are often optically
ral products such as terpenes or amino acids are frequently
active. Their biological effects may be dependent on a spe-
employed. The synthetic chemist may opt to recover the
cific configuration. Traditionally enantiomers were sep-
chiral auxiliary, in the case of reductions, or may chose to
arated by resolution, i.e., the separation of the pair of
diastereomers formed by reaction of the racemic product
and an optically pure auxiliary. The use of resolution to
FIGURE 8 Woodward synthesis from 1,3,5-cyclohexanetriol.
(a) Glyoxylic acid; (b) sodium borohydride, methanesulfonyl
FIGURE 6 Elaboration of the Corey lactone to PGF 2α . chloride; (c) potassium hydroxide; (d) potassium carbonate,
(a) (CH 3 O) 2 POCHCOC 5 H 11 ; (b) lithium triethyl borohydride; dimethoxyethane; (e) methanesulfonyl chloride; (f) potassium hy-
(c) potassium carbonate, dimethoxyethane; (d) diisobutylalu- droxide; (g) hydrogen peroxide; (h) aqueous ammonia, methanolic
minum hydride; (e) Ph 3 P CH (CH 2 ) 3 CO 2 Na. hydrogen chloride; (i) sodium nitrite, acetic acid.