Page 226 - Academic Press Encyclopedia of Physical Science and Technology 3rd Organic Chemistry
P. 226
P1: GPJ/GLT P2: GQT Final Pages
Encyclopedia of Physical Science and Technology en011-542 July 26, 2001 15:33
512 Organic Chemistry, Synthesis
1. Alkylation Reactions
Stereoselective formation of the enolate is essential for
stereoselective alkylation. An enolate may be either E or
Z using the convention that the highest priority is always
assigned to the OM group:
OM OM
R
(75)
R R
H R
Z E
Enolate geometry may be controlled by careful choice
of the deprotonation conditions. Use of sterically de-
manding lithium 2,2,6,6-tetra-methylpiperidide (LiTMP)
favors formation of the E enolate. Addition of hexam-
ethylphosphorous triamide (HMPT) to the reaction will
reverse this selectivity to favor formation of the Z eno-
late. Use of the bulky base lithium hexamethyldisilazide
(LiHMDS) will also favor formation of the Z enolate.
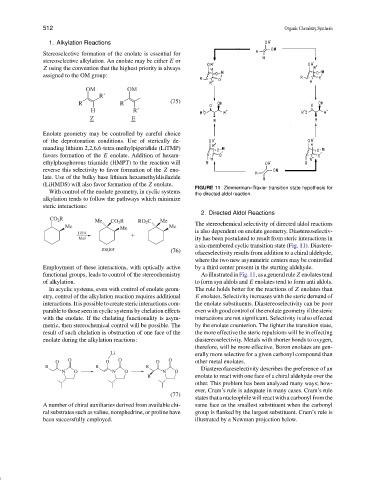
FIGURE 11 Zimmerman–Traxler transition state hypothesis for
With control of the enolate geometry, in cyclic systems
the directed aldol reaction.
alkylation tends to follow the pathways which minimize
steric interactions:
2. Directed Aldol Reactions
CO 2 R Me CO 2 R RO 2 C Me
Me Me Me The stereochemical selectivity of directed aldol reactions
LDA is also dependent on enolate geometry. Diastereoselectiv-
MeI ity has been postulated to result from steric interactions in
a six-membered cyclic transition state (Fig. 11). Diastere-
major (76) ofaceselectivity results from addition to a chiral aldehyde,
where the two new asymmetric centers may be controlled
Employment of these interactions, with optically active by a third center present in the starting aldehyde.
functional groups, leads to control of the stereochemistry AsillustratedinFig.11,asageneralrule Z enolatestend
of alkylation. to form syn aldols and E enolates tend to form anti aldols.
In acyclic systems, even with control of enolate geom- The rule holds better for the reactions of Z enolates than
etry, control of the alkylation reaction requires additional E enolates. Selectivity increases with the steric demand of
interactions. It is possible to create steric interactions com- the enolate substituents. Diastereoselectivity can be poor
parable to those seen in cyclic systems by chelation effects even with good control of the enolate geometry if the steric
with the enolate. If the chelating functionality is asym- interactions are not significant. Selectivity is also effected
metric, then stereochemical control will be possible. The by the enolate counterion. The tighter the transition state,
result of such chelation is obstruction of one face of the the more effective the steric repulsions will be in effecting
enolate during the alkylation reactions: diastereoselectivity. Metals with shorter bonds to oxygen,
therefore, will be more effective. Boron enolates are gen-
Li erally more selective for a given carbonyl compound than
O O O
O O O other metal enolates.
R R R Diastereofaceselectivity describes the preference of an
N O N O N O
R enolate to react with one face of a chiral aldehyde over the
other. This problem has been analyzed many ways; how-
ever, Cram’s rule is adequate in many cases. Cram’s rule
(77)
states that a nucleophile will react with a carbonyl from the
A number of chiral auxiliaries derived from available chi- same face as the smallest substituent when the carbonyl
ral substrates such as valine, norephedrine, or proline have group is flanked by the largest substituent. Cram’s rule is
been successfully employed. illustrated by a Newman projection below.