Page 13 - Academic Press Encyclopedia of Physical Science and Technology 3rd Analytical Chemistry
P. 13
P1: GJB Revised Pages
Encyclopedia of Physical Science and Technology En001f25 May 7, 2001 13:58
552 Analytical Chemistry
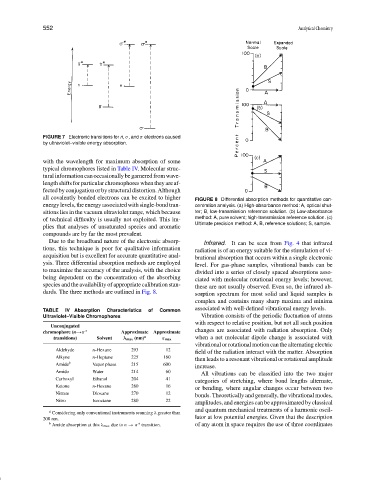
FIGURE 7 Electronic transitions for n, σ, and π electrons caused
by ultraviolet–visible energy absorption.
with the wavelength for maximum absorption of some
typical chromophores listed in Table IV. Molecular struc-
tural information can occasionally be garnered from wave-
length shifts for particular chromophores when they are af-
fected by conjugation or by structural distortion. Although
all covalently bonded electrons can be excited to higher FIGURE 8 Differential absorption methods for quantitative con-
energy levels, the energy associated with single-bond tran- centration analysis. (a) High-absorbance method: A, optical shut-
sitions lies in the vacuum ultraviolet range, which because ter; B, low-transmission reference solution. (b) Low-absorbance
of technical difficulty is usually not exploited. This im- method: A, pure solvent; high-transmission reference solution. (c)
Ultimate precision method: A, B, reference solutions; S, sample.
plies that analyses of unsaturated species and aromatic
compounds are by far the most prevalent.
Due to the broadband nature of the electronic absorp- Infrared. It can be seen from Fig. 4 that infrared
tions, this technique is poor for qualitative information radiation is of an energy suitable for the stimulation of vi-
acquisition but is excellent for accurate quantitative anal- brational absorption that occurs within a single electronic
ysis. Three differential absorption methods are employed level. For gas-phase samples, vibrational bands can be
to maximize the accuracy of the analysis, with the choice divided into a series of closely spaced absorptions asso-
being dependent on the concentration of the absorbing ciated with molecular rotational energy levels; however,
species and the availability of appropriate calibration stan- these are not usually observed. Even so, the infrared ab-
dards. The three methods are outlined in Fig. 8. sorption spectrum for most solid and liquid samples is
complex and contains many sharp maxima and minima
associated with well-defined vibrational energy levels.
TABLE IV Absorption Characteristics of Common
Ultraviolet–Visible Chromophores Vibration consists of the periodic fluctuation of atoms
with respect to relative position, but not all such position
Unconjugated
changes are associated with radiation absorption. Only
chromophore (n→π ∗ Approximate Approximate
transitions) Solvent λ max (nm) a ε max when a net molecular dipole change is associated with
vibrational or rotational motion can the alternating electric
Aldehyde n-Hexane 293 12
field of the radiation interact with the matter. Absorption
Alkyne n-Heptane 225 160
then leads to a resonant vibrational or rotational amplitude
Amide b Vapor phase 215 600
increase.
Amido Water 214 60 All vibrations can be classified into the two major
Carboxyl Ethanol 204 41 categories of stretching, where bond lengths alternate,
Ketone n-Hexane 280 16 or bending, where angular changes occur between two
Nitrate Dioxane 270 12
bonds. Theoretically and generally, the vibrational modes,
Nitro Isooctane 280 22
amplitudes, and energies can be approximated by classical
and quantum mechanical treatments of a harmonic oscil-
a Considering only conventional instruments scanning λ greater than
lator at low potential energies. Given that the description
200 nm.
b Amide absorption at this λ max due to n → σ transition. of any atom in space requires the use of three coordinates
∗