Page 168 - Academic Press Encyclopedia of Physical Science and Technology 3rd Analytical Chemistry
P. 168
P1: GQT Final
Encyclopedia of Physical Science and Technology EN006F-275 June 29, 2001 21:12
Gas Chromatography 459
The success of GC as a separation method is primar-
ily dependent on maximizing the differences in retention
times of the individual mixture components. An addi-
tional variable of such a separation process is the width
of the corresponding chromatographic peak. Whereas the
retention times are primarily dependent on the thermody-
namic properties of the separaton column, the peak width
is largely a function of the efficiency of the solute mass
transport from one phase to the other and of the kinetics of
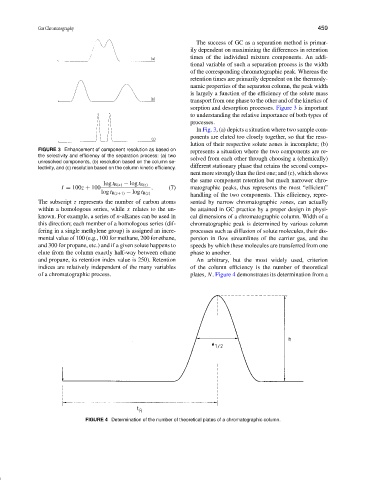
sorption and desorption processes. Figure 3 is important
to understanding the relative importance of both types of
processes.
In Fig. 3, (a) depicts a situation where two sample com-
ponents are eluted too closely together, so that the reso-
lution of their respective solute zones is incomplete; (b)
FIGURE 3 Enhancement of component resolution as based on
represents a situation where the two components are re-
the selectivity and efficiency of the separation process: (a) two
solved from each other through choosing a (chemically)
unresolved components, (b) resolution based on the column se-
different stationary phase that retains the second compo-
lectivity, and (c) resolution based on the column kinetic efficiency.
nent more strongly than the first one; and (c), which shows
the same component retention but much narrower chro-
log t R(x) − log t R(z)
I = 100z + 100 . (7) matographic peaks, thus represents the most “efficient”
log t R(z+1) − log t R(z)
handling of the two components. This efficiency, repre-
The subscript z represents the number of carbon atoms sented by narrow chromatographic zones, can actually
within a homologous series, while x relates to the un- be attained in GC practice by a proper design in physi-
known. For example, a series of n-alkanes can be used in cal dimensions of a chromatographic column. Width of a
this direction; each member of a homologous series (dif- chromatographic peak is determined by various column
fering in a single methylene group) is assigned an incre- processes such as diffusion of solute molecules, their dis-
mental value of 100 (e.g., 100 for methane, 200 for ethane, persion in flow streamlines of the carrier gas, and the
and 300 for propane, etc.) and if a given solute happens to speeds by which these molecules are transferred from one
elute from the column exactly half-way between ethane phase to another.
and propane, its retention index value is 250). Retention An arbitrary, but the most widely used, criterion
indices are relatively independent of the many variables of the column efficiency is the number of theoretical
of a chromatographic process. plates, N. Figure 4 demonstrates its determination from a
FIGURE 4 Determination of the number of theoretical plates of a chromatographic column.