Page 170 - Academic Press Encyclopedia of Physical Science and Technology 3rd Analytical Chemistry
P. 170
P1: GQT Final
Encyclopedia of Physical Science and Technology EN006F-275 June 29, 2001 21:12
Gas Chromatography 461
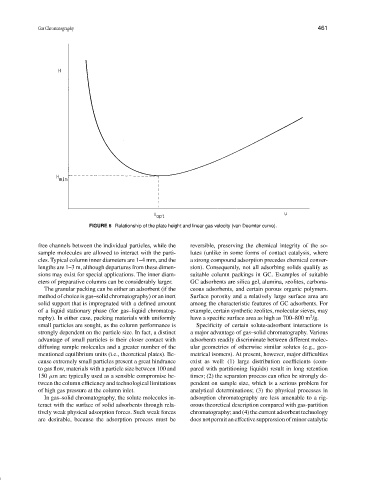
FIGURE 6 Relationship of the plate height and linear gas velocity (van Deemter curve).
free channels between the individual particles, while the reversible, preserving the chemical integrity of the so-
sample molecules are allowed to interact with the parti- lutes (unlike in some forms of contact catalysis, where
cles. Typical column inner diameters are 1–4 mm, and the a strong compound adsorption precedes chemical conver-
lengths are 1–3 m, although departures from these dimen- sion). Consequently, not all adsorbing solids qualify as
sions may exist for special applications. The inner diam- suitable column packings in GC. Examples of suitable
eters of preparative columns can be considerably larger. GC adsorbents are silica gel, alumina, zeolites, carbona-
The granular packing can be either an adsorbent (if the ceous adsorbents, and certain porous organic polymers.
method of choice is gas–solid chromatography) or an inert Surface porosity and a relatively large surface area are
solid support that is impregnated with a defined amount among the characteristic features of GC adsorbents. For
of a liquid stationary phase (for gas–liquid chromatog- example, certain synthetic zeolites, molecular sieves, may
2
raphy). In either case, packing materials with uniformly have a specific surface area as high as 700–800 m /g.
small particles are sought, as the column performance is Specificity of certain solute-adsorbent interactions is
strongly dependent on the particle size. In fact, a distinct a major advantage of gas–solid chromatography. Various
advantage of small particles is their closer contact with adsorbents readily discriminate between different molec-
diffusing sample molecules and a greater number of the ular geometries of otherwise similar solutes (e.g., geo-
mentioned equilibrium units (i.e., theoretical plates). Be- metrical isomers). At present, however, major difficulties
cause extremely small particles present a great hindrance exist as well: (1) large distribution coefficients (com-
to gas flow, materials with a particle size between 100 and pared with partitioning liquids) result in long retention
150 µm are typically used as a sensible compromise be- times; (2) the separaton process can often be strongly de-
tween the column efficiency and technological limitations pendent on sample size, which is a serious problem for
of high gas pressure at the column inlet. analytical determinations; (3) the physical processes in
In gas–solid chromatography, the solute molecules in- adsorption chromatography are less amenable to a rig-
teract with the surface of solid adsorbents through rela- orous theoretical description compared with gas-partition
tively weak physical adsorption forces. Such weak forces chromatography; and (4) the current adsorbent technology
are desirable, because the adsorption process must be does not permit an effective suppression of minor catalytic