Page 274 - Academic Press Encyclopedia of Physical Science and Technology 3rd Analytical Chemistry
P. 274
P1: GSR/GLE P2: FXY Final Pages
Encyclopedia of Physical Science and Technology EN009G-958 July 18, 2001 0:57
162 Mass Spectrometry in Forensic Science
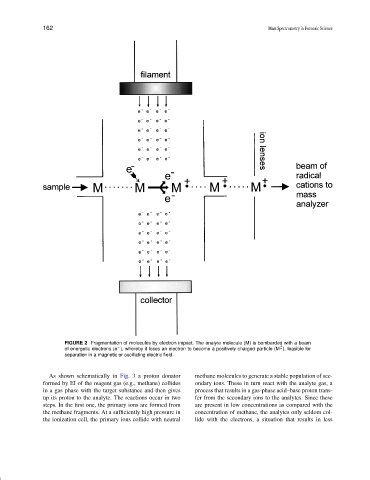
FIGURE 2 Fragmentation of molecules by electron impact. The analyte molecule (M) is bombarded with a beam
+
of energetic electrons (e ), whereby it loses an electron to become a positively charged particle (M ), feasible for
−
✉
separation in a magnetic or oscillating electric field.
As shown schematically in Fig. 3 a proton donator methane molecules to generate a stable population of sec-
formed by EI of the reagent gas (e.g., methane) collides ondary ions. These in turn react with the analyte gas, a
in a gas phase with the target substance and then gives process that results in a gas-phase acid–base proton trans-
up its proton to the analyte. The reactions occur in two fer from the secondary ions to the analytes. Since these
steps. In the first one, the primary ions are formed from are present in low concentrations as compared with the
the methane fragments. At a sufficiently high pressure in concentration of methane, the analytes only seldom col-
the ionization cell, the primary ions collide with neutral lide with the electrons, a situation that results in less