Page 275 - Academic Press Encyclopedia of Physical Science and Technology 3rd Analytical Chemistry
P. 275
P1: GSR/GLE P2: FXY Final Pages
Encyclopedia of Physical Science and Technology EN009G-958 July 18, 2001 0:57
Mass Spectrometry in Forensic Science 163
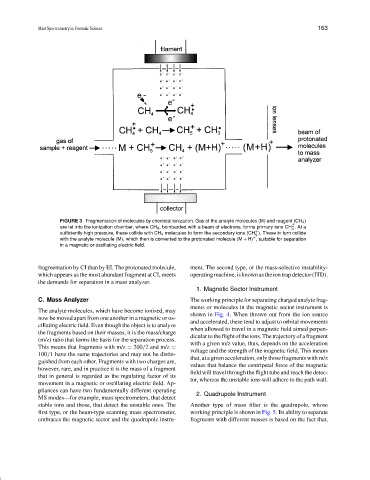
FIGURE 3 Fragmentation of molecules by chemical ionization. Gas of the analyte molecules (M) and reagent (CH 4 )
+
are let into the ionization chamber, where CH 4 , bombarded with a beam of electrons, forms primary ions CH .Ata
✉
4
sufficiently high pressure, these collide with CH 4 molecules to form the secondary ions (CH ). These in turn collide
+
5
+
with the analyte molecule (M), which then is converted to the protonated molecule (M + H) , suitable for separation
in a magnetic or oscillating electric field.
fragmentation by CI than by EI. The protonated molecule, ment. The second type, or the mass-selective instability-
which appears as the most abundant fragment at CI, meets operatingmachine,isknownastheiontrapdetector(ITD).
the demands for separation in a mass analyzer.
1. Magnetic Sector Instrument
C. Mass Analyzer The working principle for separating charged analyte frag-
ments or molecules in the magnetic sector instrument is
The analyte molecules, which have become ionized, may
shown in Fig. 4. When thrown out from the ion source
now be moved apart from one another in a magnetic or os-
and accelerated, these tend to adjust to orbital movements
cillating electric field. Even though the object is to analyze
when allowed to travel in a magnetic field aimed perpen-
the fragments based on their masses, it is the mass/charge
dicular to the flight of the ions. The trajectory of a fragment
(m/z) ratio that forms the basis for the separation process.
with a given m/z value, thus, depends on the acceleration
This means that fragments with m/z = 200/2 and m/z =
voltage and the strength of the magnetic field. This means
100/1 have the same trajectories and may not be distin-
that, at a given acceleration, only those fragments with m/z
guished from each other. Fragments with two charges are,
values that balance the centripetal force of the magnetic
however, rare, and in practice it is the mass of a fragment
field will travel through the flight tube and reach the detec-
that in general is regarded as the regulating factor of its
tor, whereas the unstable ions will adhere to the path wall.
movement in a magnetic or oscillating electric field. Ap-
pliances can have two fundamentally different operating
2. Quadrupole Instrument
MS modes—for example, mass spectrometers, that detect
stable ions and those, that detect the unstable ones. The Another type of mass filter is the quadrupole, whose
first type, or the beam-type scanning mass spectrometer, working principle is shown in Fig. 5. Its ability to separate
embraces the magnetic sector and the quadrupole instru- fragments with different masses is based on the fact that,