Page 34 - Academic Press Encyclopedia of Physical Science and Technology 3rd Analytical Chemistry
P. 34
P1: GJB Revised Pages
Encyclopedia of Physical Science and Technology En001f25 May 7, 2001 13:58
Analytical Chemistry 573
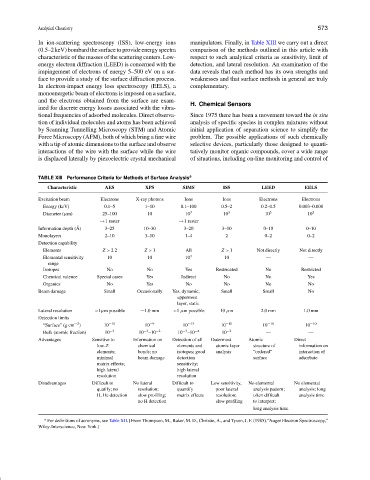
In ion-scattering spectroscopy (ISS), low-energy ions manipulators. Finally, in Table XIII we carry out a direct
(0.5–2 keV) bombard the surface to provide energy spectra comparison of the methods outlined in this article with
characteristic of the masses of the scattering centers. Low- respect to such analytical criteria as sensitivity, limit of
energy electron diffraction (LEED) is concerned with the detection, and lateral resolution. An examination of the
impingement of electrons of energy 5–500 eV on a sur- data reveals that each method has its own strengths and
face to provide a study of the surface diffraction process. weaknesses and that surface methods in general are truly
In electron-impact energy loss spectroscopy (EELS), a complementary.
monoenergetic beam of electrons is imposed on a surface,
and the electrons obtained from the surface are exam-
H. Chemical Sensors
ined for discrete energy losses associated with the vibra-
tional frequencies of adsorbed molecules. Direct observa- Since 1975 there has been a movement toward the in situ
tion of individual molecules and atoms has been achieved analysis of specific species in complex mixtures without
by Scanning Tunnelling Microscopy (STM) and Atomic initial application of separation science to simplify the
Force Microscopy (AFM), both of which bring a fine wire problem. The possible applications of such chemically
with a tip of atomic dimensions to the surface and observe selective devices, particularly those designed to quanti-
interactions of the wire with the surface while the wire tatively monitor organic compounds, cover a wide range
is displaced laterally by piezoelectric crystal mechanical of situations, including on-line monitoring and control of
TABLE XIII Performance Criteria for Methods of Surface Analysis a
Characteristic AES XPS SIMS ISS LEED EELS
Excitation beam Electrons X-ray photons Ions Ions Electrons Electrons
Energy (keV) 0.1–5 1–10 0.1–100 0.5–2 0.2–0.5 0.003–0.008
Diameter (µm) 25–100 10 10 3 10 3 10 3 10 3
→1 raster →1 raster
Information depth ( ˚ A) 3–25 10–30 3–20 3–10 0–10 0–10
Monolayers 2–10 3–10 1–4 2 0–2 0–2
Detection capability
Elements Z > 22 Z > 1 All Z > 1 Not directly Not directly
Elemental sensitivity 10 10 10 4 10 — —
range
Isotopes No No Yes Restricated No Restricted
Chemical valence Special cases Yes Indirect No No Yes
Organics No Yes No No No No
Beam damage Small Occasionally Yes, dynamic; Small Small No
uppermost
layer, static
Lateral resolution <1µm possible ∼1.0 mm <1 µm possible 10 µm 2.0 mm 1.0 mm
Detection limits
“Surface” (g cm −2 ) 10 −10 10 −9 10 −13 10 −10 10 −10 10 −10
Bulk (atomic fraction) 10 −3 10 −3 –10 −2 10 −3 –10 −4 10 −2 — —
Advantages Sensitive to Information on Detection of all Outermost Atomic Direct
low-Z chemical elements and atomic layer structure of information on
elements; bonds; no isotopes; good analysis “ordered” interaction of
minimal beam damage detection surface adsorbate
matrix effects; sensitivity;
high lateral high lateral
resolution resolution
Disadvantages Difficult to No lateral Difficult to Low sensitivity, No elemental No elemental
quatify; no resolution; quantify poor lateral analysis pattern; analysis; long
H, He detection slow profiling; matrix effects resolution; often difficult analysis time
no H detection slow profiling to interpret;
long analysis time
a For definitions of acronyms, see Table XII. [From Thompson, M., Baker, M. D., Christie, A., and Tyson, J. F. (1985).“Auger Electron Spectroscopy,”
Wiley-Interscience, New York.]