Page 30 - Academic Press Encyclopedia of Physical Science and Technology 3rd Analytical Chemistry
P. 30
P1: GJB Revised Pages
Encyclopedia of Physical Science and Technology En001f25 May 7, 2001 13:58
Analytical Chemistry 569
endothermic peaks, which correlate with weight changes,
are caused by the loss of CO to produce CaCO 3 and the
loss of CO 2 to yield CaO, respectively.
Thermal analysis of polymeric materials can be partic-
ularly fruitful for the analyst. Melting points, phase transi-
tions, pyrolysis, and curing conditions can all be gleaned
not only from the temperature positions in DTA (DSC),
but also from the width of endothermic and exothermic
peaks. In addition, it is often possible to analyze gases
that are liberated from the sample by gas chromatographs
and mass spectrometers.
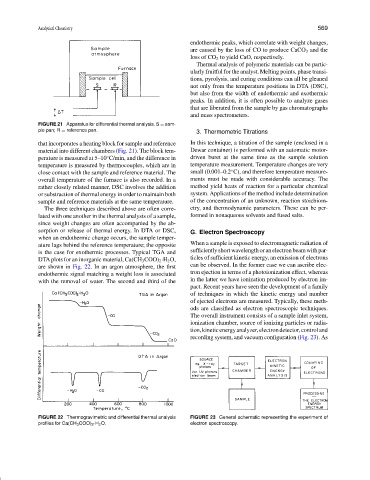
FIGURE 21 Apparatus for differential thermal analysis. S = sam-
ple pan; R = reference pan.
3. Thermometric Titrations
that incorporates a heating block for sample and reference In this technique, a titration of the sample (enclosed in a
material into different chambers (Fig. 21). The block tem- Dewar container) is performed with an automatic motor-
◦
perature is measured at 5–10 C/min, and the difference in driven buret at the same time as the sample solution
temperature is measured by thermocouples, which are in temperature measurement. Temperature changes are very
◦
close contact with the sample and reference material. The small (0.001–0.2 C), and therefore temperature measure-
overall temperature of the furnace is also recorded. In a ments must be made with considerable accuracy. The
rather closely related manner, DSC involves the addition method yield heats of reaction for a particular chemical
or substraction of thermal energy in order to maintain both system. Applications of the method include determination
sample and reference materials at the same temperature. of the concentration of an unknown, reaction stoichiom-
The three techniques described above are often corre- etry, and thermodynamic parameters. These can be per-
lated with one another in the thermal analysis of a sample, formed in nonaqueous solvents and fused salts.
since weight changes are often accompanied by the ab-
sorption or release of thermal energy. In DTA or DSC, G. Electron Spectroscopy
when an endothermic change occurs, the sample temper-
ature lags behind the reference temperature; the opposite When a sample is exposed to electromagnetic radiation of
is the case for exothermic processes. Typical TGA and sufficiently short wavelength or an electron beam with par-
DTA plots for an inorganic material, Ca(CH 3 COO) 2 ·H 2 O, ticles of sufficient kinetic energy, an emission of electrons
are shown in Fig. 22. In an argon atmosphere, the first can be observed. In the former case we can ascribe elec-
endothermic signal matching a weight loss is associated tron ejection in terms of a photoionization effect, whereas
with the removal of water. The second and third of the in the latter we have ionization produced by electron im-
pact. Recent years have seen the development of a family
of techniques in which the kinetic energy and number
of ejected electrons are measured. Typically, these meth-
ods are classified as electron spectroscopic techniques.
The overall instrument consists of a sample inlet system,
ionization chamber, source of ionizing particles or radia-
tion,kineticenergyanalyzer,electrondetector,controland
recording system, and vacuum configuration (Fig. 23). As
FIGURE 22 Thermogravimetric and differential thermal analysis FIGURE 23 General schematic representing the experiment of
profiles for Ca(CH 3 COO) 2 ·H 2 O. electron spectroscopy.