Page 31 - Academic Press Encyclopedia of Physical Science and Technology 3rd Analytical Chemistry
P. 31
P1: GJB Revised Pages
Encyclopedia of Physical Science and Technology En001f25 May 7, 2001 13:58
570 Analytical Chemistry
with the mass spectrometer, an electrostatic field can be
usedtofocuselectronsofcertainenergyatanexitslitready
for counting (detection). The experimental plot of number
of electrons vs their kinetic energy is called an electron
energy spectrum. The individual techniques in electron
spectroscopy are classified according to either the method
of inducing ionization or the nature of the process that
accompanies the emission of electrons.
1. Vacuum Ultraviolet
Photoelectron Spectroscopy
In vacuum ultraviolet photoelectron spectroscopy (UPS),
the sample atom or molecule is exposed to radiation in the
vacuum ultraviolet region of the electromagnetic spec-
trum. A readily available source of radiation is the he-
lium discharge lamp, which produces a sharp HeI line at
21.2 eV. Since the energy required for photoionization of
sets of valence electrons is in the vicinity of 6 eV to this
energy, we obtain a polyenergetic emission of electrons
described by the Einstein relation
E n = hν − I n ,
where I n is the ionization energy of the nth species of
electron and E n is the kinetic energy of the bunch of elec-
trons ejected by a photon of energy hν. Usually, we form
an experimental plot of numbers of electrons vs ioniza-
tion potential (the photoelectron spectrum), since hν is a
known quantity and we wish to know I n .
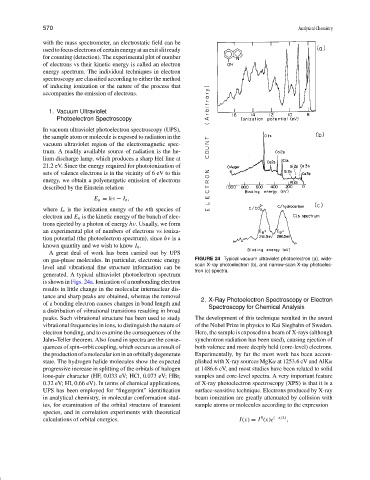
A great deal of work has been carried out by UPS
on gas-phase molecules. In particular, electronic energy FIGURE 24 Typical vacuum ultraviolet photoelectron (a), wide-
scan X-ray photoelectron (b), and narrow-scan X-ray photoelec-
level and vibrational fine structure information can be
tron (c) spectra.
generated. A typical ultraviolet photoelectron spectrum
is shown in Figs. 24a. Ionization of a nonbonding electron
results in little change in the molecular internuclear dis-
tance and sharp peaks are obtained, whereas the removal
2. X-Ray Photoelectron Spectroscopy or Electron
of a bonding electron causes changes in bond length and
Spectroscopy for Chemical Analysis
a distribution of vibrational transitions resulting in broad
peaks. Such vibrational structure has been used to study The development of this technique resulted in the award
vibrational frequencies in ions, to distinguish the nature of of the Nobel Prize in physics to Kai Siegbahn of Sweden.
electron bonding, and to examine the consequences of the Here, the sample is exposed to a beam of X-rays (although
Jahn–Teller theorem. Also found in spectra are the conse- synchrotron radiation has been used), causing ejection of
quences of spin–orbit coupling, which occurs as a result of both valence and more deeply held (core-level) electrons.
theproductionofamolecularioninanorbitallydegenerate Experimentally, by far the most work has been accom-
state. The hydrogen halide molecules show the expected plished with X-ray sources MgKα at 1253.6 eV and AlKα
progressive increase in splitting of the orbitals of halogen at 1486.6 eV, and most studies have been related to solid
lone-pair character (HF, 0.033 eV; HCl, 0.073 eV; HBr, samples and core-level spectra. A very important feature
0.32 eV; HI, 0.66 eV). In terms of chemical applications, of X-ray photoelectron spectroscopy (XPS) is that it is a
UPS has been employed for “fingerprint” identification surface-sensitive technique. Electrons produced by X-ray
in analytical chemistry, in molecular conformation stud- beam ionization are greatly attenuated by collision with
ies, for examination of the orbital structure of transient sample atoms or molecules according to the expression
species, and in correlation experiments with theoretical
0
calculations of orbital energies. I(x) = I (x)e (−x/λ) ,