Page 435 - Academic Press Encyclopedia of Physical Science and Technology 3rd Analytical Chemistry
P. 435
P1: GLQ Final pages
Encyclopedia of Physical Science and Technology EN012C-568 July 26, 2001 15:32
Photoelectron Spectroscopy 85
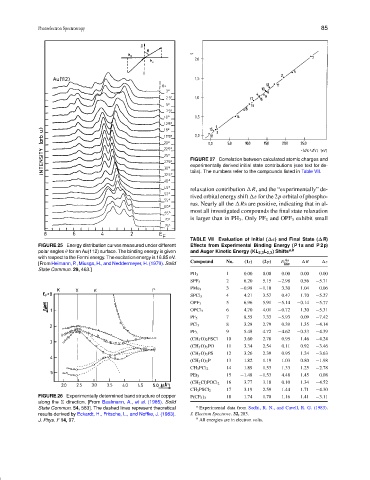
FIGURE 27 Correlation between calculated atomic charges and
experimentally derived initial state contributions (see text for de-
tails). The numbers refer to the compounds listed in Table VII.
relaxation contribution R, and the “experimentally” de-
rived orbital energy shift ε for the 2p orbital of phospho-
rus. Nearly all the Rs are positive, indicating that in al-
most all investigated compounds the final state relaxation
is larger than in PH 3 . Only PF 3 and OPF 3 exhibit small
TABLE VII Evaluation of Initial (∆ε) and Final State (∆R)
FIGURE 25 Energy distribution curves measured under different Effects from Experimental Binding Energy (P 1s and P 2p)
polar angles θ for an Au(112) surface. The binding energy is given and Auger Kinetic Energy (KL 2,3 L 2,3 ) Shifts a,b
with respect to the Fermi energy. The excitation energy is 16.85 eV.
Compound No. (1s) (2p) E Au R ε
[From Heimann, P., Miusga, H., and Neddermeyer, H. (1979). Solid kin
State Commun. 29, 463.]
PH 3 1 0.00 0.00 0.00 0.00 0.00
SPF 3 2 6.20 5.15 −2.98 0.56 −5.71
PMe 3 3 −0.98 −1.10 3.30 1.04 0.06
SPCl 3 4 4.21 3.57 0.47 1.70 −5.27
OPF 3 5 6.96 5.91 −5.14 −0.14 −5.77
OPCl 3 6 4.70 4.01 −0.72 1.30 −5.31
PF 5 7 8.55 7.33 −5.93 0.09 −7.42
PCl 3 8 3.28 2.79 0.39 1.35 −4.14
PF 3 9 5.48 4.72 −4.62 −0.33 −4.39
(CH 3 O) 2 PSCl 10 3.60 2.78 0.95 1.46 −4.24
(CH 3 O) 3 PO 11 3.34 2.54 0.11 0.92 −3.46
(CH 3 O) 3 PS 12 3.26 2.39 0.95 1.24 −3.63
(CH 3 O) 3 P 13 1.82 1.19 1.03 0.80 −1.98
CH 3 PCl 2 14 1.89 1.53 1.33 1.25 −2.78
PEt 3 15 −1.48 −1.53 4.48 1.45 0.08
(CH 2 Cl)POCl 2 16 3.77 3.18 0.10 1.34 −4.52
CH 3 PSCl 2 17 3.19 2.59 1.44 1.71 −4.30
FIGURE 26 Experimentally determined band structure of copper P(CF 3 ) 3 18 1.74 1.70 1.16 1.41 −3.11
along the direction. [From Baalmann, A., et al. (1985). Solid
State Commun. 54, 583]. The dashed lines represent theoretical a Experimental data from Sodhi, R. N., and Cavell, R. G. (1983).
results derived by Eckardt, H., Fritsche, L., and Noffke, J. (1983). J. Electron Spectrosc. 32, 283.
J. Phys. F 14, 97. b All energies are in electron volts.