Page 431 - Academic Press Encyclopedia of Physical Science and Technology 3rd Analytical Chemistry
P. 431
P1: GLQ Final pages
Encyclopedia of Physical Science and Technology EN012C-568 July 26, 2001 15:32
Photoelectron Spectroscopy 81
well-separated bands are observed with an intensity ratio
of approximately 1:3:1. These bands can be correlated to
the five highest occupied orbitals of trans-stilbene, which
are all π orbitals. This situation, where it is possible to
assign several bands at the beginning of the PE spectrum
of a larger molecule, is usually met in cases where the up-
permost occupied orbitals are energetically well separated
from the main body of the valence orbitals. Among these
special orbitals are n orbitals, π orbitals, and the d orbitals
of transition metal complexes.
Very often it is not the electronic structure of a single
compound that is studied by PES. In most cases, it is the
change in electronic structure caused by some variations
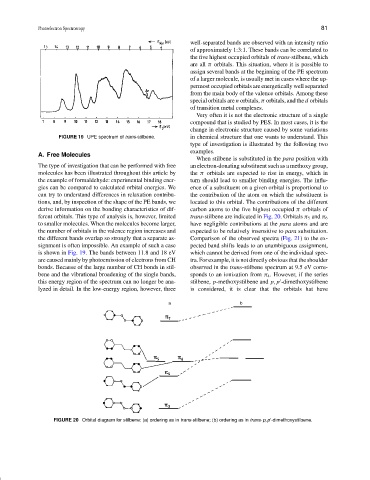
FIGURE 19 UPE spectrum of trans-stilbene. in chemical structure that one wants to understand. This
type of investigation is illustrated by the following two
examples.
A. Free Molecules
When stilbene is substituted in the para position with
The type of investigation that can be performed with free an electron-donating substituent such as a methoxy group,
molecules has been illustrated throughout this article by the π orbitals are expected to rise in energy, which in
the example of formaldehyde: experimental binding ener- turn should lead to smaller binding energies. The influ-
gies can be compared to calculated orbital energies. We ence of a substituent on a given orbital is proportional to
can try to understand differences in relaxation contribu- the contribution of the atom on which the substituent is
tions, and, by inspection of the shape of the PE bands, we located to this orbital. The contributions of the different
derive information on the bonding characteristics of dif- carbon atoms to the five highest occupied π orbitals of
ferent orbitals. This type of analysis is, however, limited trans-stilbene are indicated in Fig. 20. Orbitals π 5 and π 6
to smaller molecules. When the molecules become larger, have negligible contributions at the para atoms and are
the number of orbitals in the valence region increases and expected to be relatively insensitive to para substitution.
the different bands overlap so strongly that a separate as- Comparison of the observed spectra (Fig. 21) to the ex-
signment is often impossible. An example of such a case pected band shifts leads to an unambiguous assignment,
is shown in Fig. 19. The bands between 11.8 and 18 eV which cannot be derived from one of the individual spec-
are caused mainly by photoemission of electrons from CH tra. For example, it is not directly obvious that the shoulder
bonds. Because of the large number of CH bonds in stil- observed in the trans-stilbene spectrum at 9.5 eV corre-
bene and the vibrational broadening of the single bands, sponds to an ionization from π 4 . However, if the series
this energy region of the spectrum can no longer be ana- stilbene, p-methoxystilbene and p,p -dimethoxystilbene
lyzed in detail. In the low-energy region, however, three is considered, it is clear that the orbitals hat have
FIGURE 20 Orbital diagram for stilbene: (a) ordering as in trans-stilbene; (b) ordering as in trans-p,p -dimethoxystilbene.