Page 22 - Academic Press Encyclopedia of Physical Science and Technology 3rd BioChemistry
P. 22
P1: ZCK Final Pages
Encyclopedia of Physical Science and Technology EN005G-231 June 15, 2001 20:46
Enzyme Mechanisms 629
Materials that bind to the enzyme either at the active site
or at a distal site and slow the turnover of the enzyme but
are not themselves transformed act as inhibitors. These
compounds may or may not be structurally similar to the
substrate; nevertheless, their binding, particularly at the
active site, often provides important complexes for struc-
ture determination. The most commonly studied type of
inhibition is termed competitive, which means that the
substrate and the inhibitor compete directly for the active
site of the enzyme. The effect of this type of inhibitor on
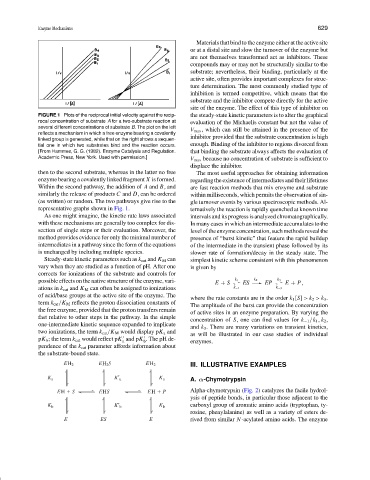
FIGURE 1 Plots of the reciprocal initial velocity against the recip- the steady-state kinetic parameters is to alter the graphical
rocal concentration of substrate A for a two-substrate reaction at evaluation of the Michaelis constant but not the value of
several different concentrations of substrate B. The plot on the left
V max , which can still be attained in the presence of the
reflects a mechanism in which a free enzyme bearing a covalently
inhibitor provided that the substrate concentration is high
linked group is generated, while that on the right shows a sequen-
tial one in which two substrates bind and the reaction occurs. enough. Binding of the inhibitor to regions divorced from
[From Hammes, G. G. (1982). Enzyme Catalysis and Regulation. that binding the substrate always affects the evaluation of
Academic Press, New York. Used with permission.] V max because no concentration of substrate is sufficient to
displace the inhibitor.
then to the second substrate, whereas in the latter no free The most useful approaches for obtaining information
enzyme bearing a covalently linked fragment X is formed. regarding the existence of intermediates and their lifetimes
Within the second pathway, the addition of A and B, and are fast reaction methods that mix enzyme and substrate
similarly the release of products C and D, can be ordered within milliseconds, which permits the observation of sin-
(as written) or random. The two pathways give rise to the gle turnover events by various spectroscopic methods. Al-
representative graphs shown in Fig. 1. ternatively the reaction is rapidly quenched at known time
As one might imagine, the kinetic rate laws associated intervals and its progress is analyzed chromatographically.
with these mechanisms are generally too complex for dis- In many cases in which an intermediate accumulates to the
section of single steps or their evaluation. Moreover, the leveloftheenzymeconcentration,suchmethodsrevealthe
method provides evidence for only the minimal number of presence of “burst kinetic” that feature the rapid buildup
intermediates in a pathway since the form of the equations of the intermediate in the transient phase followed by its
is unchanged by including multiple species. slower rate of formation/decay in the steady state. The
Steady-state kinetic parameters such as k cat and K M can simplest kinetic scheme consistent with this phenomenon
vary when they are studied as a function of pH. After one is given by
corrects for ionizations of the substrate and controls for
possible effects on the native structure of the enzyme, vari- E + S k 1 ES k 2 EP k 3 E + P,
ations in k cat and K M can often be assigned to ionizations k −1 k −3
of acid/base groups at the active site of the enzyme. The
where the rate constants are in the order k 1 [S] > k 2 > k 3 .
term k cat /K M reflects the proton dissociation constants of
The amplitude of the burst can provide the concentration
the free enzyme, provided that the proton transfers remain
of active sites in an enzyme preparation. By varying the
fast relative to other steps in the pathway. In the simple
concentration of S, one can find values for k −1 /k 1 , k 2 ,
one-intermediate kinetic sequence expanded to implicate
and k 3 . There are many variations on transient kinetics,
two ionizations, the term k cat /K M would display pK a and
as will be illustrated in our case studies of individual
pK b ; the term k cat would reflect pK and pK . The pH de-
a b enzymes.
pendence of the k cat parameter affords information about
the substrate-bound state.
EH 2 E H 2 S E H 2 III. ILLUSTRATIVE EXAMPLES
A. α-Chymotrypsin
K a K′ a K a
E H S E HS EH P Alpha-chymotrypsin (Fig. 2) catalyzes the facile hydrol-
ysis of peptide bonds, in particular those adjacent to the
carboxyl group of aromatic amino acids (tryptophan, ty-
K b K′ b K b
rosine, phenylalanine) as well as a variety of esters de-
E ES E rived from similar N-acylated amino acids. The enzyme