Page 24 - Academic Press Encyclopedia of Physical Science and Technology 3rd BioChemistry
P. 24
P1: ZCK Final Pages
Encyclopedia of Physical Science and Technology EN005G-231 June 15, 2001 20:46
Enzyme Mechanisms 631
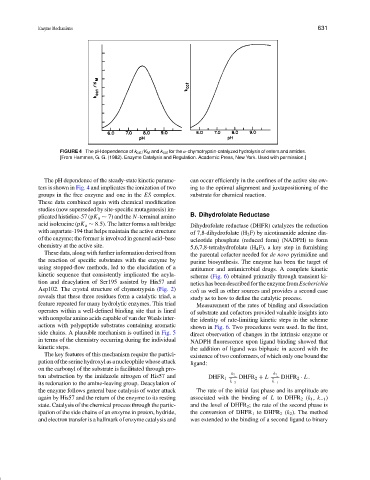
FIGURE 4 The pH dependence of k cat /K M and k cat for the α-chymotrypsin-catalyzed hydrolysis of esters and amides.
[From Hammes, G. G. (1982). Enzyme Catalysis and Regulation. Academic Press, New York. Used with permission.]
The pH dependence of the steady-state kinetic parame- can occur efficiently in the confines of the active site ow-
ters is shown in Fig. 4 and implicates the ionization of two ing to the optimal alignment and juxtapositioning of the
groups in the free enzyme and one in the ES complex. substrate for chemical reaction.
These data combined again with chemical modification
studies (now superseded by site-specific mutagenesis) im-
B. Dihydrofolate Reductase
plicated histidine-57 (pK a ∼ 7) and the N-terminal amino
acid isoleucine (pK a ∼ 8.5). The latter forms a salt bridge Dihydrofolate reductase (DHFR) catalyzes the reduction
with aspartate-194 that helps maintain the active structure of 7,8-dihydrofolate (H 2 F) by nicotinamide adenine din-
of the enzyme; the former is involved in general acid–base ucleotide phosphate (reduced form) (NADPH) to form
chemistry at the active site. 5,6,7,8-tetrahydrofolate (H 4 F), a key step in furnishing
These data, along with further information derived from the parental cofactor needed for de novo pyrimidine and
the reaction of specific substrates with the enzyme by purine biosynthesis. The enzyme has been the target of
using stopped-flow methods, led to the elucidation of a antitumor and antimicrobial drugs. A complete kinetic
kinetic sequence that consistently implicated the acyla- scheme (Fig. 6) obtained primarily through transient ki-
tion and deacylation of Ser195 assisted by His57 and netics has been described for the enzyme from Escherichia
Asp102. The crystal structure of chymotrypsin (Fig. 2) coli as well as other sources and provides a second case
reveals that these three residues form a catalytic triad, a study as to how to define the catalytic process.
feature repeated for many hydrolytic enzymes. This triad Measurement of the rates of binding and dissociation
operates within a well-defined binding site that is lined of substrate and cofactors provided valuable insights into
with nonpolar amino acids capable of van der Waals inter- the identity of rate-limiting kinetic steps in the scheme
actions with polypeptide substrates containing aromatic shown in Fig. 6. Two procedures were used. In the first,
side chains. A plausible mechanism is outlined in Fig. 5 direct observation of changes in the intrinsic enzyme or
in terms of the chemistry occurring during the individual NADPH fluorescence upon ligand binding showed that
kinetic steps. the addition of ligand was biphasic in accord with the
The key features of this mechanism require the partici- existence of two conformers, of which only one bound the
pation of the serine hydroxyl as a nucleophile whose attack ligand:
on the carbonyl of the substrate is facilitated through pro-
ton abstraction by the imidazole nitrogen of His57 and DHFR 1 k 2 DHFR 2 + L k 1 DHFR 2 · L.
its redonation to the amine-leaving group. Deacylation of k −2 k −1
the enzyme follows general base catalysis of water attack The rate of the initial fast phase and its amplitude are
again by His57 and the return of the enzyme to its resting associated with the binding of L to DHFR 2 (k 1 , k −1 )
state. Catalysis of the chemical process through the partic- and the level of DHFR 2 ; the rate of the second phase is
ipation of the side chains of an enzyme in proton, hydride, the conversion of DHFR 1 to DHFR 2 (k 2 ). The method
and electron transfer is a hallmark of enzyme catalysis and was extended to the binding of a second ligand to binary