Page 23 - Academic Press Encyclopedia of Physical Science and Technology 3rd BioChemistry
P. 23
P1: ZCK Final Pages
Encyclopedia of Physical Science and Technology EN005G-231 June 15, 2001 20:46
630 Enzyme Mechanisms
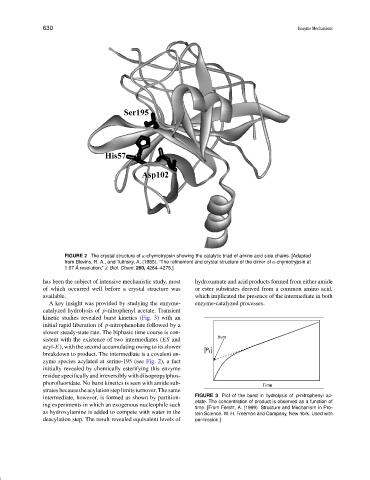
FIGURE 2 The crystal structure of α-chymotrypsin showing the catalytic triad of amino acid side chains. [Adapted
from Blevins, R. A., and Tulinsky, A. (1985). “The refinement and crystal structure of the dimer of α-chymotrypsin at
1.67 ˚ A resolution,” J. Biol. Chem. 260, 4264–4275.]
has been the subject of intensive mechanistic study, most hydroxamate and acid products formed from either amide
of which occurred well before a crystal structure was or ester substrates derived from a common amino acid,
available. which implicated the presence of the intermediate in both
A key insight was provided by studying the enzyme- enzyme-catalyzed processes.
catalyzed hydrolysis of p-nitrophenyl acetate. Transient
kinetic studies revealed burst kinetics (Fig. 3) with an
initial rapid liberation of p-nitrophenolate followed by a
slower steady-state rate. The biphasic time course is con-
sistent with the existence of two intermediates (ES and
acyl-E), with the second accumulating owing to its slower
breakdown to product. The intermediate is a covalent en-
zyme species acylated at serine-195 (see Fig. 2), a fact
initially revealed by chemically esterifying this enzyme
residue specifically and irreversibly with diisopropylphos-
phorofluoridate. No burst kinetics is seen with amide sub-
stratesbecausetheacylationsteplimitsturnover.Thesame
intermediate, however, is formed as shown by partition- FIGURE 3 Plot of the burst in hydrolysis of p-nitrophenyl ac-
etate. The concentration of product is observed as a function of
ing experiments in which an exogenous nucleophile such
time. [From Fersht, A. (1999). Structure and Mechanism in Pro-
as hydroxylamine is added to compete with water in the tein Science. W. H. Freeman and Company, New York. Used with
deacylation step. The result revealed equivalent levels of permission.]