Page 6 - Academic Press Encyclopedia of Physical Science and Technology 3rd BioChemistry
P. 6
P1: FYD Revised Pages
Encyclopedia of Physical Science and Technology EN002H-54 May 17, 2001 20:22
102 Bioenergetics
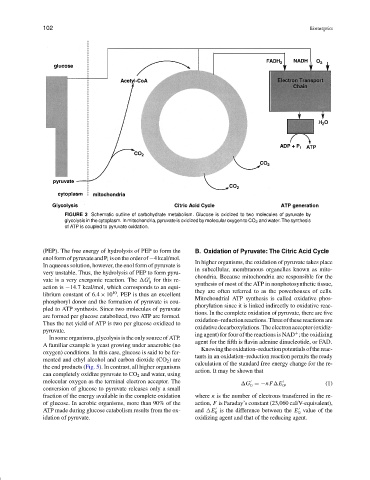
FIGURE 3 Schematic outline of carbohydrate metabolism. Glucose is oxidized to two molecules of pyruvate by
glycolysis in the cytoplasm. In mitochondria, pyruvate is oxidized by molecular oxygen to CO 2 and water. The synthesis
of ATP is coupled to pyruvate oxidation.
(PEP). The free energy of hydrolysis of PEP to form the B. Oxidation of Pyruvate: The Citric Acid Cycle
enolformofpyruvateandP i isontheorderof−4kcal/mol.
In higher organisms, the oxidation of pyruvate takes place
In aqueous solution, however, the enol form of pyruvate is
in subcellular, membranous organelles known as mito-
very unstable. Thus, the hydrolysis of PEP to form pyru-
chondria. Because mitochondria are responsible for the
vate is a very exergonic reaction. The G for this re-
0 synthesis of most of the ATP in nonphotosynthetic tissue,
action is −14.7 kcal/mol, which corresponds to an equi- they are often referred to as the powerhouses of cells.
10
librium constant of 6.4 × 10 . PEP is thus an excellent
Mitochondrial ATP synthesis is called oxidative phos-
phosphoryl donor and the formation of pyruvate is cou-
phorylation since it is linked indirectly to oxidative reac-
pled to ATP synthesis. Since two molecules of pyruvate
tions. In the complete oxidation of pyruvate, there are five
are formed per glucose catabolized, two ATP are formed.
oxidation–reductionreactions.Threeofthesereactionsare
Thus the net yield of ATP is two per glucose oxidized to
oxidativedecarboxylations.Theelectronacceptor(oxidiz-
pyruvate.
+
ing agent) for four of the reactions is NAD ; the oxidizing
In some organisms, glycolysis is the only source of ATP.
agent for the fifth is flavin adenine dinucleotide, or FAD.
A familiar example is yeast growing under anaerobic (no
Knowingtheoxidation–reductionpotentialsofthereac-
oxygen) conditions. In this case, glucose is said to be fer-
tants in an oxidation–reduction reaction permits the ready
mented and ethyl alcohol and carbon dioxide (CO 2 ) are
calculation of the standard free energy change for the re-
the end products (Fig. 5). In contrast, all higher organisms
action. It may be shown that
can completely oxidize pyruvate to CO 2 and water, using
molecular oxygen as the terminal electron acceptor. The
G =−nF E , (1)
0
0
conversion of glucose to pyruvate releases only a small
fraction of the energy available in the complete oxidation where n is the number of electrons transferred in the re-
of glucose. In aerobic organisms, more than 90% of the action, F is Faraday’s constant (23,060 cal/V-equivalent),
ATP made during glucose catabolism results from the ox- and E is the difference between the E value of the
0 0
idation of pyruvate. oxidizing agent and that of the reducing agent.