Page 7 - Academic Press Encyclopedia of Physical Science and Technology 3rd BioChemistry
P. 7
P1: FYD Revised Pages
Encyclopedia of Physical Science and Technology EN002H-54 May 17, 2001 20:22
Bioenergetics 103
FADH 2 is −38 kcal/mol. These two strongly exergonic re-
actions provide the energy for the endergonic synthesis of
ATP.
The details of carbon metabolism in the citric acid cy-
cle are beyond the scope of this article. In brief, pyruvate
is first oxidatively decarboxylated to yield CO 2 , NADH,
and an acetyl group attached in an ester linkage to a thiol
on a large molecule, known as coenzyme A, or CoA. (See
Fig. 2.) Acetyl CoA condenses with a four-carbon dicar-
boxylic acid to form the tricarboxylic acid citrate. Free
CoA is also a product (Fig. 6). A total of four oxidation–
reductionreactions,twoofwhichareoxidativedecarboxy-
lations, take place, which results in the generation of the
three remaining NADH molecules and one molecule of
FADH 2 . The citric acid cycle is a true cycle. For each
two-carbon acetyl moiety oxidized in the cycle, two CO 2
molecules are produced and the four-carbon dicarboxylic
acid with which acetyl CoA condenses is regenerated.
The mitochondrial inner membrane (Fig. 7) contains
proteins that act in concert to catalyze NADH and FADH 2
oxidation by molecular oxygen. [See reactions (2) and (3)
above.] These reactions are carried out in many small steps
by proteins that are integral to the membrane and that un-
dergo oxidation–reduction. These proteins make up what
is called the mitochondrial electron transport chain. Com-
ponents of the chain include iron proteins (cytochromes
and iron–sulfur proteins), flavoproteins (proteins that con-
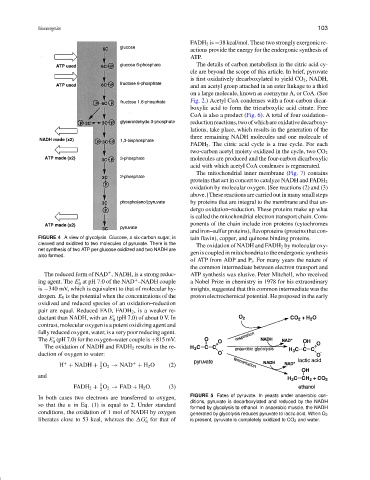
FIGURE 4 A view of glycolysis. Glucose, a six-carbon sugar, is tain flavin), copper, and quinone binding proteins.
cleaved and oxidized to two molecules of pyruvate. There is the
The oxidation of NADH and FADH 2 by molecular oxy-
net synthesis of two ATP per glucose oxidized and two NADH are
gen is coupled in mitochondria to the endergonic synthesis
also formed.
of ATP from ADP and P i . For many years the nature of
the common intermediate between electron transport and
+
The reduced form of NAD , NADH, is a strong reduc- ATP synthesis was elusive. Peter Mitchell, who received
ing agent. The E at pH 7.0 of the NAD –NADH couple a Nobel Prize in chemistry in 1978 for his extraordinary
+
0
is −340 mV, which is equivalent to that of molecular hy- insights, suggested that this common intermediate was the
drogen. E 0 is the potential when the concentrations of the proton electrochemical potential. He proposed in the early
oxidized and reduced species of an oxidation–reduction
pair are equal. Reduced FAD, FADH 2 , is a weaker re-
ductant than NADH, with an E (pH 7.0) of about 0 V. In
0
contrast, molecular oxygen is a potent oxidizing agent and
fully reduced oxygen, water, is a very poor reducing agent.
The E (pH 7.0) for the oxygen–water couple is +815 mV.
0
The oxidation of NADH and FADH 2 results in the re-
duction of oxygen to water:
1
+
H + NADH + O 2 → NAD + H 2 O (2)
+
2
and
1
FADH 2 + O 2 → FAD + H 2 O. (3)
2
In both cases two electrons are transferred to oxygen, FIGURE 5 Fates of pyruvate. In yeasts under anaerobic con-
ditions, pyruvate is decarboxylated and reduced by the NADH
so that the n in Eq. (1) is equal to 2. Under standard
formed by glycolysis to ethanol. In anaerobic muscle, the NADH
conditions, the oxidation of 1 mol of NADH by oxygen
generated by glycolysis reduces pyruvate to lactic acid. When O 2
liberates close to 53 kcal, whereas the G for that of is present, pyruvate is completely oxidized to CO 2 and water.
0