Page 9 - Academic Press Encyclopedia of Physical Science and Technology 3rd BioChemistry
P. 9
P1: FYD Revised Pages
Encyclopedia of Physical Science and Technology EN002H-54 May 17, 2001 20:22
Bioenergetics 105
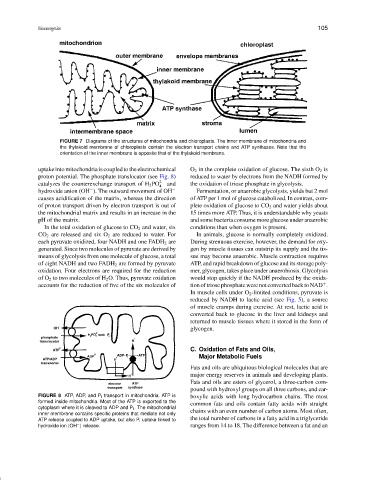
FIGURE 7 Diagrams of the structures of mitochondria and chloroplasts. The inner membrane of mitochondria and
the thylakoid membrane of chloroplasts contain the electron transport chains and ATP synthases. Note that the
orientation of the inner membrane is opposite that of the thylakoid membrane.
uptake into mitochondria is coupled to the electrochemical O 2 in the complete oxidation of glucose. The sixth O 2 is
proton potential. The phosphate translocator (see Fig. 8) reduced to water by electrons from the NADH formed by
catalyzes the counterexchange transport of H 2 PO 2− and the oxidation of triose phosphate in glycolysis.
4
hydroxide anion (OH ). The outward movement of OH − Fermentation, or anaerobic glycolysis, yields but 2 mol
−
causes acidification of the matrix, whereas the direction of ATP per 1 mol of glucose catabolized. In contrast, com-
of proton transport driven by electron transport is out of plete oxidation of glucose to CO 2 and water yields about
the mitochondrial matrix and results in an increase in the 15 times more ATP. Thus, it is understandable why yeasts
pH of the matrix. and some bacteria consume more glucose under anaerobic
In the total oxidation of glucose to CO 2 and water, six conditions than when oxygen is present.
CO 2 are released and six O 2 are reduced to water. For In animals, glucose is normally completely oxidized.
each pyruvate oxidized, four NADH and one FADH 2 are During strenuous exercise, however, the demand for oxy-
generated. Since two molecules of pyruvate are derived by gen by muscle tissues can outstrip its supply and the tis-
means of glycolysis from one molecule of glucose, a total sue may become anaerobic. Muscle contraction requires
of eight NADH and two FADH 2 are formed by pyruvate ATP, and rapid breakdown of glucose and its storage poly-
oxidation. Four electrons are required for the reduction mer, glycogen, takes place under anaerobiosis. Glycolysis
of O 2 to two molecules of H 2 O. Thus, pyruvate oxidation would stop quickly if the NADH produced by the oxida-
+
accounts for the reduction of five of the six molecules of tion of triose phosphate were not converted back to NAD .
In muscle cells under O 2 -limited conditions, pyruvate is
reduced by NADH to lactic acid (see Fig. 5), a source
of muscle cramps during exercise. At rest, lactic acid is
converted back to glucose in the liver and kidneys and
returned to muscle tissues where it stored in the form of
glycogen.
C. Oxidation of Fats and Oils,
Major Metabolic Fuels
Fats and oils are ubiquitous biological molecules that are
major energy reserves in animals and developing plants.
Fats and oils are esters of glycerol, a three-carbon com-
pound with hydroxyl groups on all three carbons, and car-
FIGURE 8 ATP, ADP, and P i transport in mitochondria. ATP is boxylic acids with long hydrocarbon chains. The most
formed inside mitochondria. Most of the ATP is exported to the common fats and oils contain fatty acids with straight
cytoplasm where it is cleaved to ADP and P i . The mitochondrial
inner membrane contains specific proteins that mediate not only chains with an even number of carbon atoms. Most often,
ATP release coupled to ADP uptake, but also P i uptake linked to the total number of carbons in a fatty acid in a triglyceride
−
hydroxide ion (OH ) release. ranges from 14 to 18. The difference between a fat and an