Page 10 - Academic Press Encyclopedia of Physical Science and Technology 3rd Polymer
P. 10
P1: FPP 2nd Revised Pages
Encyclopedia of Physical Science and Technology EN002C-64 May 19, 2001 20:39
214 Biopolymers
the folding of more and more proteins is studied, we re-
alize that most proteins can be described in terms of a
small number of regular packing arrangements of helices
and/or β-structures. Thus, proteins can be considered to
be constructed of modules, each module being a structural
domain of mainly α-helices and/or β-structure. One pro-
tein differs from another, then, in the number, type, and
relative arrangement of domains within the molecule, and
in the detailed arrangement of amino acid side groups, R
groups, on the molecular surface.
The tertiary structure of a protein molecule encom-
passes the overall folding of polypeptide chains, where,
if more than one chain is present, the chains are linked
by covalent bonds—most often disulfide bonds (as in II).
However, some proteins exist where the molecules consist
of several separate polypeptide chains; such chains would
be held together in the molecule by weaker bonds such as
hydrogen and ionic bonds, hydrophobic interactions, and
van der Waals forces and each chain can act as a separate
subunit of the protein molecule. These proteins are said to
possess quaternary structure (i.e., specific arrangements
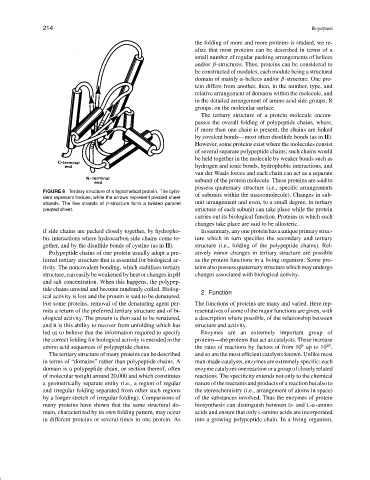
FIGURE 6 Tertiary structure of a hypothetical protein. The cylin-
of subunits within the macromolecule). Changes in sub-
ders represent helices, while the arrows represent pleated sheet
unit arrangement and even, to a small degree, in tertiary
strands. The five strands of β-structure form a twisted parallel
pleated sheet. structure of each subunit can take place while the protein
carries out its biological function. Proteins in which such
changes take place are said to be allosteric.
if side chains are packed closely together, by hydropho- In summary, any one protein has a unique primary struc-
bic interactions where hydrocarbon side chains come to- ture which in turn specifies the secondary and tertiary
gether, and by the disulfide bonds of cystine (as in II). structure (i.e., folding of the polypeptide chains). Rel-
Polypeptide chains of one protein usually adopt a pre- atively minor changes in tertiary structure are possible
ferred tertiary structure that is essential for biological ac- as the protein functions in a living organism. Some pro-
tivity. The noncovalent bonding, which stabilizes tertiary teins also possess quaternary structure which may undergo
structure, can easily be weakened by heat or changes in pH changes associated with biological activity.
and salt concentration. When this happens, the polypep-
tide chains unwind and become randomly coiled. Biolog-
2. Function
ical activity is lost and the protein is said to be denatured.
For some proteins, removal of the denaturing agent per- The functions of proteins are many and varied. Here rep-
mits a return of the preferred tertiary structure and of bi- resentatives of some of the major functions are given, with
ological activity. The protein is then said to be renatured, a description where possible, of the relationship between
and it is this ability to recover from unfolding which has structure and activity.
led us to believe that the information required to specify Enzymes are an extremely important group of
the correct folding for biological activity is encoded in the proteins—the proteins that act as catalysts. These increase
20
8
amino acid sequences of polypeptide chains. the rates of reactions by factors of from 10 up to 10 ,
The tertiary structure of many proteins can be described and so are the most efficient catalysts known. Unlike most
in terms of “domains” rather than polypeptide chains. A man-made catalysts, enzymes are extremely specific; each
domain is a polypeptide chain, or section thereof, often enzyme catalyzes one reaction or a group of closely related
of molecular weight around 20,000 and which constitutes reactions. The specificity extends not only to the chemical
a geometrically separate entity (i.e., a region of regular nature of the reactants and products of a reaction but also to
and irregular folding separated from other such regions the stereochemistry (i.e., arrangement of atoms in space)
by a longer stretch of irregular folding). Comparisons of of the substances involved. Thus the enzymes of protein
many proteins have shown that the same structural do- biosynthesis can distinguish between D- and L-α-amino
main, characterized by its own folding pattern, may occur acids and ensure that only L-amino acids are incorporated
in different proteins or several times in one protein. As into a growing polypeptide chain. In a living organism,