Page 8 - Academic Press Encyclopedia of Physical Science and Technology 3rd Polymer
P. 8
P1: FPP 2nd Revised Pages
Encyclopedia of Physical Science and Technology EN002C-64 May 19, 2001 20:39
212 Biopolymers
The α-helix structure of proteins was first proposed
by Pauling and Corey and was confirmed later by X-ray
diffraction studies. For L-α-amino acids a right-handed
helix, as shown in Fig. 5a, is energetically more favorable
than a left-handed helix. The helix is stabilized by hydro-
gen bonds from a backbone NH group to oxygen of a
C O group of the fourth amino acid residue further back
on the chain. The side groups of amino acids project out-
ward from the helix. Proline residues (see Table I) in a
polypeptide chain lack the hydrogen necessary for hydro-
gen bonding; a helix-stabilizing hydrogen bond cannot
form, and so the presence of proline tends to disrupt
α-helices.
FIGURE 3 Arrangement in space of atoms of an extended poly-
peptide. Atoms 1–6 are almost coplanar, as are atoms 6–11, but Nl
N CHR 1 C 2 C
in a different plane from 1–6.
H O H O
Hydrogen
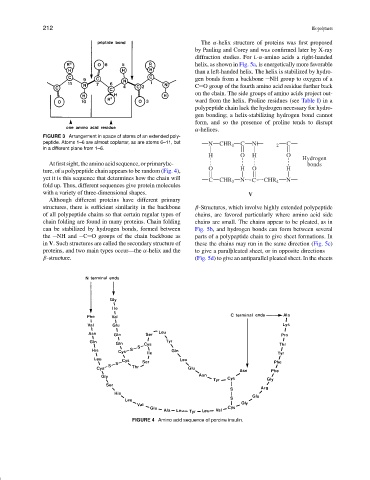
At first sight, the amino acid sequence, or primaryluc- bonds
O H O H
ture, of a polypeptide chain appears to be random (Fig. 4),
yet it is this sequence that determines how the chain will C CHR 3 N C CHR 4 N
fold up. Thus, different sequences give protein molecules
with a variety of three-dimensional shapes. V
Although different proteins have different primary
structures, there is sufficient similarity in the backbone β-Structures, which involve highly extended polypeptide
of all polypeptide chains so that certain regular types of chains, are favored particularly where amino acid side
chain folding are found in many proteins. Chain folding chains are small. The chains appear to be pleated, as in
can be stabilized by hydrogen bonds, formed between Fig. 5b, and hydrogen bonds can form between several
the NH and C O groups of the chain backbone as parts of a polypeptide chain to give sheet formations. In
in V. Such structures are called the secondary structure of these the chains may run in the same direction (Fig. 5c)
proteins, and two main types occur—the α-helix and the to give a parallpleated sheet, or in opposite directions
β-structure. (Fig. 5d) to give an antiparallel pleated sheet. In the sheets
FIGURE 4 Amino acid sequence of porcine insulin.