Page 6 - Academic Press Encyclopedia of Physical Science and Technology 3rd Polymer
P. 6
P1: FPP 2nd Revised Pages
Encyclopedia of Physical Science and Technology EN002C-64 May 19, 2001 20:39
210 Biopolymers
in only L-α-amino acids becoming incorporated into the
polymers. Nineteen different amino acids of general for-
mula H 2 N-CHR-COOH can be used as monomers in pro-
tein synthesis, and these vary only in the nature of the
side group R. A list of possible side groups is shown in
Table I. In addition an imino acid, proline, with a cyclic
side chain and having an NH group instead of the NH 2
of the amino acids, can act as a protein monomer. Some
side chains (R groups) can be chemically modified after
incorporation of the amino acid into a protein, and three
of these modifications are also shown in Table I. Thus, hy-
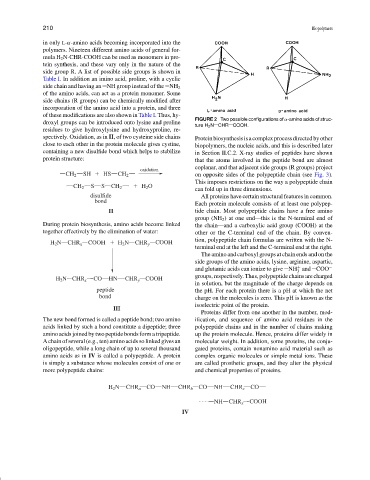
FIGURE 2 Two possible configurations of α-amino acids of struc-
droxyl groups can be introduced onto lysine and proline
ture H 2 N CHR COOH.
residues to give hydroxylysine and hydroxyproline, re-
spectively. Oxidation, as in II, of two cysteine side chains Proteinbiosynthesisisacomplexprocessdirectedbyother
close to each other in the protein molecule gives cystine, biopolymers, the nucleic acids, and this is described later
containing a new disulfide bond which helps to stabilize in Section II.C.2. X-ray studies of peptides have shown
protein structure: that the atoms involved in the peptide bond are almost
coplanar, and that adjacent side groups (R groups) project
oxidation
SH HS
CH 2 CH 2 on opposite sides of the polypeptide chain (see Fig. 3).
This imposes restrictions on the way a polypeptide chain
S S H 2 O
CH 2 CH 2
can fold up in three dimensions.
disulfide All proteins have certain structural features in common.
bond
Each protein molecule consists of at least one polypep-
II tide chain. Most polypeptide chains have a free amino
group (NH 2 ) at one end—this is the N-terminal end of
During protein biosynthesis, amino acids become linked the chain—and a carboxylic acid group (COOH) at the
together effectively by the elimination of water: other or the C-terminal end of the chain. By conven-
tion, polypeptide chain formulas are written with the N-
H 2 N CHR x COOH H 2 N CHR y COOH
terminal end at the left and the C-terminal end at the right.
The amino and carboxyl groups at chain ends and on the
side groups of the amino acids, lysine, arginine, aspartic,
+
and glutamic acids can ionize to give NH and COO −
3
groups, respectively. Thus, polypeptide chains are charged
H 2 N CHR x CO HN CHR y COOH
in solution, but the magnitude of the charge depends on
peptide the pH. For each protein there is a pH at which the net
bond charge on the molecules is zero. This pH is known as the
isoelectric point of the protein.
III
Proteins differ from one another in the number, mod-
The new bond formed is called a peptide bond; two amino ification, and sequence of amino acid residues in the
acids linked by such a bond constitute a dipeptide; three polypeptide chains and in the number of chains making
amino acids joined by two peptide bonds form a tripeptide. up the protein molecule. Hence, proteins differ widely in
A chain of several (e.g., ten) amino acids so linked gives an molecular weight. In addition, some proteins, the conju-
oligopeptide, while a long chain of up to several thousand gated proteins, contain nonamino acid material such as
amino acids as in IV is called a polypeptide. A protein complex organic molecules or simple metal ions. These
is simply a substance whose molecules consist of one or are called prosthetic groups, and they alter the physical
more polypeptide chains: and chemical properties of proteins.
H 2 N CHR a CO NH CHR b CO NH CHR c CO
NH CHR x COOH
IV