Page 9 - Academic Press Encyclopedia of Physical Science and Technology 3rd Polymer
P. 9
P1: FPP 2nd Revised Pages
Encyclopedia of Physical Science and Technology EN002C-64 May 19, 2001 20:39
Biopolymers 213
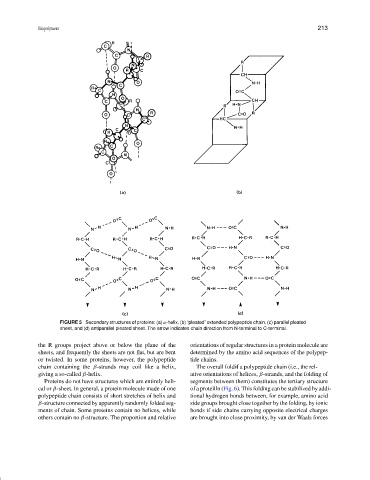
FIGURE 5 Secondary structures of proteins: (a) α-helix, (b) “pleated” extended polypeptide chain, (c) parallel pleated
sheet, and (d) antiparallel pleated sheet. The arrow indicates chain direction from N-terminal to C-terminal.
the R groups project above or below the plane of the orientations of regular structures in a protein molecule are
sheets, and frequently the sheets are not flat, but are bent determined by the amino acid sequences of the polypep-
or twisted. In some proteins, however, the polypeptide tide chains.
chain containing the β-strands may coil like a helix, The overall folditf a polypeptide chain (i.e., the rel-
giving a so-called β-helix. ative orientations of helices, β-strands, and the folding of
Proteins do not have structures which are entirely heli- segments between them) constitutes the tertiary structure
cal or β-sheet. In general, a protein molecule made of one of a proteilln (Fig. 6). This folding can be stabilized by addi-
polypeptide chain consists of short stretches of helix and tional hydrogen bonds between, for example, amino acid
β-structure connected by apparently randomly folded seg- side groups brought close together by the folding, by ionic
ments of chain. Some proteins contain no helices, while bonds if side chains carrying opposite electrical charges
others contain no β-structure. The proportion and relative are brought into close proximity, by van der Waals forces